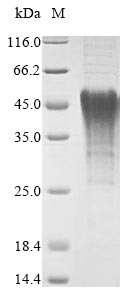
Recombinant Mouse Bnip3 is produced using an in vitro E.coli expression system in the lab, containing a partial sequence that spans amino acids 50 to 187. The protein includes an N-terminal 6xHis tag, which makes purification and detection much easier. Based on SDS-PAGE analysis, the product appears to have a purity level above 90%, suggesting it should be reliable for research work. This is strictly for research purposes - not for any human or animal treatments or diagnostic testing.
Bnip3 belongs to the Bcl-2 family and seems to play an important role in controlling both apoptosis and autophagy pathways. It's particularly known for its connection to mitochondrial dynamics and how cells respond when oxygen levels drop. Because of its function in programmed cell death, Bnip3 has become a key protein for scientists studying cell survival and death mechanisms. Research into this protein may provide valuable insights into various normal and disease-related processes.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Mouse Bnip3 is a mitochondrial membrane protein that requires precise folding, transmembrane domain integration, and homodimerization for its pro-apoptotic activity. The in vitro E. coli expression system (cell-free) cannot provide the necessary membrane environment for this transmembrane protein. The partial fragment (50-187aa) lacks critical N-terminal and transmembrane domains, and the N-terminal 6xHis-tag may further interfere with proper folding. While cell-free systems can produce soluble proteins, the probability of correct folding with functional activity for this membrane-associated protein fragment is very low.
1. Antibody Development and Validation
This application is highly suitable as antibody development relies on antigenic sequence recognition. The fragment provides specific epitopes within the 50-187aa region for antibody production, independent of native folding.
2. Structural and Biophysical Characterization Studies
These studies can characterize the physical properties of this specific fragment, but cannot reflect the native Bnip3 structure. Techniques like circular dichroism can analyze secondary structure content, but results will describe a soluble fragment rather than the membrane-integrated protein.
Final Recommendation & Action Plan
The in vitro E. coli expression system is fundamentally unsuitable for producing a functional version of this transmembrane protein. This Bnip3 fragment is primarily suitable for antibody development (Application 1) and basic biophysical characterization of the fragment itself (Application 2). Avoid protein interaction studies and functional binding studies entirely due to the high probability of misfolding and lack of membrane context. For reliable Bnip3 research, use a full-length protein expressed in mammalian systems with proper membrane integration.