Anti-idiotypic antibodies
1. What are anti-idiotypic antibodies?
Anti-idiotypic antibodies are a type of antibody that can specifically bind to the unique site, known as the paratope, on another antibody. The paratope is a special region on the antibody molecule, also referred to as the variable region or antigen-binding site. Idiotopes are composed of multiple antigenic determinants, and each determinant represents a unique site. These antigenic determinants or idiotopes can be found in the heavy chain variable region, the light chain variable region, or on the surface composed of both chains.
Even for antibodies originating from the same species and individual, their immunogenicity can vary significantly. Idiotypes are unique antigen-specific markers present on each antibody molecule, and their binding sites are called idiotope. Each Fab segment of the antibody has approximately 5-6 idiotopes, and they are located in the variable region. Idiotypes can stimulate the production of corresponding antibodies, including anti-idiotypic antibodies, in heterologous, homologous, or even the same individual, hence the term "anti-idiotypic antibodies."
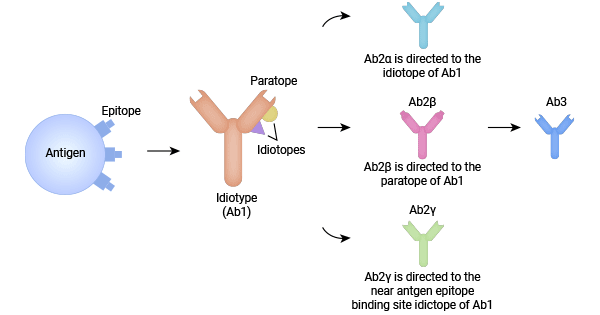
Anti-idiotypic antibodies originate from the idiotype network theory of Ab1-Ab2-Ab3, which was proposed by Niels Jerne in 1974. This network theory comprises idiotypes (Ab1), anti-idiotypic antibodies (also known as Ab2), and antigen-presenting cells. According to the network theory, the presence of epitopes or antigenic determinants stimulates the production of antigen-specific antibodies (Ab1), which then induce the production of anti-idiotypic antibodies (Ab2) to maintain balance. Ab2 can be classified into Ab2α, Ab2β, or Ab2γ (Figure 1) based on their antigen-binding sites. More recently, two additional categories, Ab2δ and Ab2ε, have been identified. Ab2α targets the idiotope of Ab1, while Ab2γ targets the idiotope near the antigen-binding site of Ab1. Ab2β carries an internal image binding site complementary to the idiotope of Ab1 and mimics Ab1's structure. Ab2 can stimulate the production of anti-anti-idiotypic antibodies (Ab3), which have a similar binding capability to Ab1, forming a mutually regulating and interconnected idiotype network. This network theory allows the production of anti-idiotypic antibodies to regulate the secretion of autoantibodies by neutralizing and inhibiting them, ultimately contributing to the prevention of autoimmune diseases [1].
Figure 1. The idiotype network theory of Ab1-Ab2-Ab3
2. Classification of Anti-Idiotypic Antibodies
Anti-idiotypic antibodies are classified into three types based on their interaction with antibody drugs and the detection method.
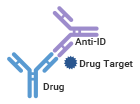
The first type is the Antigen Blocking Anti-Idiotypic Antibody, also known as Antigen Neutralizing Anti-Idiotypic Antibody. In this type, the antibody drug's antigenic site overlaps with the idiotope, leading to competition between the target antigen of the antibody drug and the anti-idiotypic antibody. As a result, this type of anti-idiotypic antibody can only detect free antibody drugs.
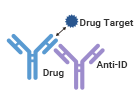
The second type of anti-idiotypic antibody is called Non-Blocking (or Antigen Non-Neutralizing) Anti-Idiotypic Antibody. In this case, the antibody drug's antigenic site and idiotope do not overlap. Therefore, the anti-idiotypic antibody and the antigen can simultaneously bind to the antibody drug without affecting each other's binding capacity. These antibodies lack specificity in the antibody-binding region, allowing therapeutic antibodies to bind to their target antigens. This type of anti-idiotypic antibody is used to detect the total amount of antibody drugs.
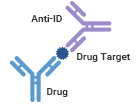
The third type of anti-idiotypic antibody is Complex-Specific Anti-Idiotypic Antibody, also known as Drug Target Complex Anti-Idiotypic Antibody. This type of antibody is highly specific and only recognizes the antibody-target complex. It does not bind to unbound antibodies or unbound target antigens. Consequently, this type of anti-idiotypic antibody is exclusively capable of detecting bound antibody drugs.
| Type |
Antigen-Neutralizing |
Antigen-Non-Neutralizing |
Drug-Target Complex |
| Application Range |
PK Testing |
Immunogenicity Evaluation |
|
| Characteristics |
Antibody Binding Site Specificity |
Non-Antibody Binding Site Characteristics |
Drug-Target Complex Specificity |
| Antigen Binding |
Inhibitory and Neutralizing |
Non-Inhibitory |
Non-Inhibitory |
| Application |
Target Blockage, Detection of Free Drug |
Target Non-Blockage, Detection of Total Drug |
Target Non-Blockage, Detection of Bound Drug |
| Schematic Diagram |
|
|
|
| Advantages |
1. Single epitope
2. High specificity
3. Good batch-to-batch stability
|
1. Can simulate real conditions in blood samples
2. Relatively short preparation period
3. Low cost
|
|
3. Applications of Anti-Idiotypic Antibodies
Anti-idiotypic antibodies are further categorized into monoclonal and polyclonal antibodies, with slight differences in their specific applications.
Monoclonal antibodies are characterized by strong specificity and uniformity, making them commonly used in preclinical pharmacological research and idiotype antibody assays during clinical phases. Monoclonal anti-idiotypic antibodies can specifically recognize the unique sites of biopharmaceuticals, allowing for the quantification of antibody drug levels in animal and human serum in pharmacokinetic studies.
Polyclonal antibodies offer higher coverage, closely simulating the situation of anti-drug antibodies, and are typically recommended as positive controls for anti-drug antibody detection and immunogenicity assessments.
Currently, anti-idiotypic antibodies find applications in the immunogenicity analysis of antibody drugs, preclinical research of therapeutic antibodies, clinical development of anti-drug antibodies, ligand neutralization tests, control in antibody blocking assays, as well as pharmacokinetic (PK) and pharmacodynamic (PD) analysis of antibody drugs.
● Immunogenicity Analysis
Immunogenicity analysis is of paramount importance in the development of biopharmaceuticals. Virtually all biopharmaceutical products, such as proteins, antibodies, peptide-conjugated drugs, or oligonucleotides, induce an immune response within the body, resulting in the production of anti-drug antibodies (ADAs). Immunogenicity testing methods offer several advantages, including:
- High Sensitivity (<100 ng/mL): Capable of detecting ADA at very low concentrations.
- Coverage of All ADA Subtypes (IgG, IgM, IgE, etc.): Able to detect various ADA subtypes.
- Utilization of Anti-ID Antibodies as Positive Control Reagents: Establishing methodological validation using Anti-ID antibodies.
- Establishment of Negative Controls (Non-dosed individuals, healthy individuals, target disease groups): Setting up control groups for comparison.
These advantages make immunogenicity analysis an essential tool in assessing the potential immune response to biopharmaceutical products during their development.
Anti-idiotypic antibodies are highly specific ADAs. Polyclonal antibodies can closely simulate the actual scenario in blood samples and offer advantages of shorter preparation cycles and lower costs. Therefore, in most cases where the presence of ADAs in patient samples is analyzed, polyclonal anti-idiotypic antibodies are typically used as positive controls.
● Pharmacokinetic (PK) Analysis
Anti-idiotypic antibodies also serve as critical reagents for pharmacokinetic (PK) analysis. In preclinical and clinical studies, PK analysis is employed to assess the dosing and toxicity of antibody drugs.
Monoclonal anti-idiotypic antibodies, known for their strong specificity, are used as test reagents for PK analysis to detect the levels of antibody drugs in human or animal sera. Specifically, clinicians need to determine the rates of drug absorption, distribution, bioavailability, and elimination in different patient groups. To achieve this goal, researchers must be able to track and quantify various forms of antibody therapeutic drugs in patient serum, blood, urine, or other bodily fluids at different time points following drug administration. By employing primarily monoclonal anti-idiotypic antibodies, various forms of antibody therapy drugs can be easily tracked and quantified in patient serum, blood, urine, or other bodily fluids.
CUSABIO offers tailored services for developing and preparing anti-idiotypic antibodies, depending on the type of antibodies to be detected. We provide services for antigen-blocking type, non-neutralizing type, and drug-target complex type. Additionally, to meet diverse customer needs, we offer a range of services, including antigen preparation, monoclonal anti-idiotypic antibodies, polyclonal anti-idiotypic antibodies, and PK Testing assays, among others.
| Custom Anti-Idiotypic Antibody Products |
Customer Provides |
Preparation Period(Business Days) |
Delivered by CUSABIO |
Application Range |
| Anti-Fab Mouse Monoclonal Antibodies |
10mg full-length positive antibody |
80-100 |
- ① 3-5 hybridoma cell lines;
- ② 2mg monoclonal antibody (purity above 95% as determined by SDS-PAGE);
- ③ Comprehensive test reports.
|
PK Testing |
| Anti-Scfv Mouse Monoclonal Antibodies |
scFv Antibody Sequences |
110-130 |
- ① 3-5 hybridoma cell lines;
- ② 2mg monoclonal antibody (purity above 95% as determined by SDS-PAGE);
- ③ Comprehensive test reports.
|
PK Testing |
| Anti-idiotype Rabbit Polyclonal Antibodies Customization |
30mg full-length positive antibody |
45-55 |
- ① 1mL pre-immune serum;
- ② 2mg rabbit polyclonal antibodies (guaranteed titer 1:64000, purity above 95% as determined by
SDS-PAGE);
- ③ Comprehensive test reports.
|
Immunogenicity Evaluation |
References
[1].Pan S Y, Chia Y C, Yee H R, et al. Immunomodulatory potential of anti-idiotypic antibodies for the treatment of autoimmune diseases[J]. Future science OA, 2020, 7(2): FSO648.