1. What is Hippo Signaling Pathway?
The Hippo signaling pathway, also known as the Salvador/Warts/Hippo (SWH) pathway, consists of a group of conserved kinases and controls organ size through the regulation of cell proliferation and apoptosis in animals. The Hippo signaling pathway can inhibit cell growth. The name of the pathway is derived from one of its key signaling components-the protein kinase Hippo (Hpo) in fruit fly. Tissue overgrowth is the major cause for mutations in this gene. In this review, we Primary introduce the mammals’ Hippo signaling pathway. In mammals, the membrane protein receptor, located in upstream of the Hippo signaling pathway, senses the extracellular environmental growth-inhibitory signal and then acts on the downstream effectors YAP and TAZ after kinase cascade phosphorylation reactions.
2. The Members of Hippo Signaling Pathway
The Hippo signaling pathway is highly conserved through evolution. All core components of the Hippo pathway that identified in the Drosophila have direct orthologs in mammals(Table 1)[1].
Table 1. Hippo Signaling Pathway Components in Drosophila and Human
|
Drosophila
|
Human ortholog(s)
|
The Role of Protein in Hippo Signaling Pathway
|
|
Dachsous (Ds)
|
DCHS1, DCHS2
|
Atypical cadherin that may act as a ligand for the Fat receptor.
|
|
Fat (Ft)
|
FAT1, FAT2, FAT3, FAT4(FATJ)
|
Atypical cadherin that may act as a receptor for the Hippo pathway.
|
|
Expanded (Ex)
|
FRMD6/Willin
|
FERM domain-containing apical protein that associates with Kibra and Mer as an upstream regulator of the core kinase cascade.
|
|
Dachs (Dachs)
|
|
Unconventional myosin that can bind Wts, promoting its degradation.
|
|
Kibra (Kibra)
|
WWC1
|
WW domain-containing apical protein that associates with Ex and Mer as an upstream regulator of the core kinase cascade.
|
|
Merlin (Mer)
|
NF2
|
FERM domain-containing apical protein that associates with Ex and Kibra as an upstream regulator of the core kinase cascade.
|
|
Hippo (Hpo)
|
MST1, MST2
|
Sterile-20-type kinase that phosphorylates and activates Wts.
|
|
Salvador (Sav)
|
WW45 (SAV1)
|
WW domain-containing protein that may act as a scaffold protein, facilitating Warts phosphorylation by Hippo.
|
|
Warts (Wts)
|
LATS1, LATS2
|
Nuclear DBF-2-related kinase that phosphorylates and inactivates Yki.
|
|
Mob as tumor suppressor (Mats)
|
MOBKL1A, MOBKL1B
|
Kinase that associates with Wts to potentiate its catalytic activity.
|
|
Yorkie (Yki)
|
YAP, TAZ
|
Transcriptional coactivator that binds to Sd in its active, unphosphorylated form to activate expression of transcriptional targets that promote cell growth, cell proliferation, and prevent apoptosis
|
|
Scalloped (Sd)
|
TEAD1, TEAD2, TEAD3, TEAD4
|
Transcription factor that binds Yki to regulate target gene expression
|
* Content of the Table 1 is derived from Wikipedia
3. The Function of Hippo Signaling Pathway(immune cells, osteoclast formation)
In Drosophila and mammals, studies over the past decade have revealed that Hippo signaling pathway controls organ size by regulating cell proliferation, apoptosis, and stem cell self-renewal[2]. Dysregulation of this pathway contributes to massive overgrowth of tissue. The Hippo signaling pathway is highly conserved and limits organ size by phosphorylating and inhibiting the transcription co-activators YAP and TAZ in mammals(ortholog of Drosophila Yki), key regulators of proliferation and apoptosis. Emerging evidence shows that the Hippo signaling pathway plays a critical role in regulating osteoclast formation and cancer, and has important function in immune cells[3][4]. In this part, we conclude the function of the Hippo signaling pathway in organ size control and osteoclast formation.
a. The Function of Hippo Signaling Pathway in Organ Size Control
The Hippo pathway was initially thought to limit organ size by inhibiting proliferation and promoting apoptosis. A kinase cascade is a core of the Hippo signaling pathway. Mst1/2 (ortholog of Drosophila Hippo) kinases and SAV1 form a complex to phosphorylate LATS1/2. Activated LATS1/2 kinases in turn phosphorylate and inhibit the transcription co-activators YAP and TAZ, which are two major downstream effectors of the Hippo pathway. On the contrary, dephosphorylated YAP/TAZ translocate into the nucleus and interact with TEAD1-4 and other transcription factors to induce expression of genes that promote cell proliferation and inhibit apoptosis. Functions of the Hippo pathway in organ size determination have been confirmed in mouse models. For example, liver-specific overexpression of YAP leads to enlarged livers that return to their normal size after cessation of YAP expression[5][6].
b. The Function of Hippo Signaling Pathway in Osteoclast Formation
A subtle balance between osteoblastic bone formation and osteoclastic bone resorption plays a key role in keeping bone homeostasis. Emerging evidences shows that this process is regulated by the Hippo signaling pathway including crucial regulatory molecules RASSF2, NF2, MST1/2, SAV1, LATS1/2, Ajuba, MOB1, YAP and TAZ. Among of them, RASSF2, NF2 and MST1/2 genes regulate pre-osteoclast proliferation, and SAV1, LATS1/2, YAP and TAZ genes regulate osteoclast differentiation[7][8][9][10]. In addition, regulated bone apoptosis genes include RASSF, MST and TAZ, which are the downstream genes of the Hippo signaling pathway[11][12][13].
The common cellular process of osteoclasts includes pre-osteoclast proliferation, osteoclast differentiation, and apoptosis which involves different molecular cascades. Amount of evidence has revealed that Hippo-pathway may play a key role in these processes by the interactions between the NF-κB-, MAPK-, and calcium-signaling pathways. In the Hippo- and NF-κB-signaling pathways, RASSF2 and MST2 suppress IKK and I-κBα activities respectively which block NF-κB signaling pathway. In the Hippo- and MAPK-signaling pathways, Ajuba activates TRAF6, YAP/TAZ/TEAD activates ERK, JNK, p38, and AP1. Moreover, in the Hippo- and calcium-signaling pathways, YAP activates CREB and TEADs-dependent reduces calcineurin activity, thus, inhibits NFATc1[7].
4. YAP and TAZ: a Nexus for Hippo Signaling and Others
Yes-associated protein (YAP)/transcriptional coactivator with a PDZ-binding domain (TAZ) is the prime mediators of the Hippo signaling pathway and has a direct orthologs in Drosophila, Yorkie. The Hippo kinase cascade is the primary regulator of YAP/TAZ by phosphorylating YAP/TAZ and inhibiting their nuclear activities[14]. Considerable research data indicate that YAP/TAZ, the key regulator of the Hippo signaling pathway, as a nexus and integrator for multiple prominent pathways and signaling organelles that play key roles in the control of cell fate and tissue regeneration, such as Wnt, G protein-coupled receptor (GPCR), epidermal growth factor (EGF), bone morphogenetic protein (BMP)/transforming growth factor beta (TGFβ), and Notch pathways. Here, I have organized several pathways that are currently well-studied.
a. YAP/TAZ and Wnt signaling
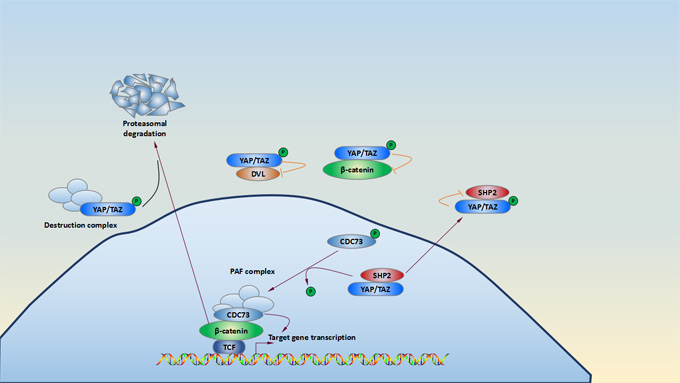
There are two forms YAP/YAZ involving in the Hippo signaling pathway. One form is phosphorylated YAP/TAZ, which is phosphorylated directly by activated LATS1/2 kinases. In turn, multiple serine residues of YAP and TAZ is phosphorylated and leads to cytoplasmic retention of YAP/TAZ via a 14-3-3 interaction or be degraded by proteasomal. Another form of TAP/YAZ is hypophosphorylated YAP/TAZ. When the kinase module of the Hippo signaling pathway is inactivated, hypophosphorylated YAP/TAZ translocate into the nucleus and induce related target gene expression.
β-catenin as a regulator, plays a key role in mediating Wnt signaling pathway. Cytoplasmic YAP/TAZ detain β-catenin and in some cases recruit β-TrCP to the destruction complex[15][16]. This change leads to nuclear β-catenin downregulation and then inhibits Wnt signaling. Cytoplasmic YAP and TAZ also negatively regulate Wnt signaling by detaining β-catenin and disheveled (DVL)[17]. The tyrosine phosphatase SHP2, as well as YAP and TAZ, shuttles between the nucleus and the cytoplasm. When it is located in nuclear, it promotes β-catenin-dependent transcription[18][19]. SHP2 is translocated in cytoplasm by interaction with phosphorylated YAP and TAZ. YAP and TAZ control nuclear and cytoplasmic localization of SHP2 and its function.
b. TGFβ and BMP signaling
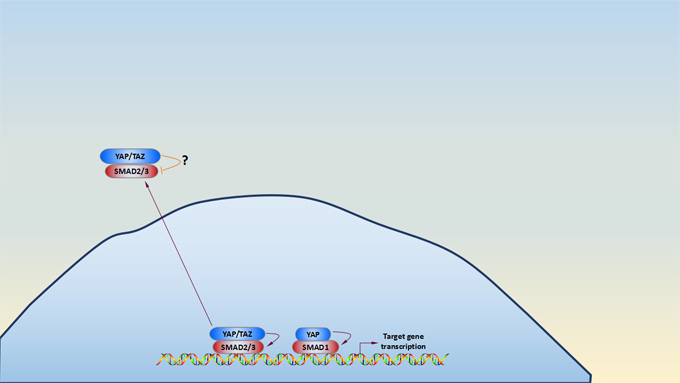
Accumulating evidence shows that YAP and TAZ utilizes some transcription factors (TFs), and some contextual transcriptional responses which are mediated by additional TFs such as TEADs, SMADs, p63, RUNX, and PAX, which diversifies the transcriptional output of the Hippo pathway[20][21][22]. Phosphorylation and activation of SMAD is induced by TGFβ/BMP binding to its serine-threonine kinase receptor[23][24]. As shown in the picture of Hippo signaling pathway, cytoplasmic YAP and TAZ sequester SMAD2/3 in the cytoplasm and then inhibit SMAD2/3-mediated signaling, but these mechanisms seem not to be widespread and somewhat controversial[25]. Nuclear YAP activates SMAD1-mediated transcription. Once TGFβ signal activation, nuclear YAP/TAZ and SMAD2/3 form a complex, and TEA domain family (TEAD) transcription factors, coordinating a protumorigenic transcriptional program. YAP and TAZ also directly promote transcription of several receptors and ligands to mediate endocrine and paracrine signal crosstalk and potential feedback loops[26][27].
*Pictures are derived from Carsten Gram Hansen’s research[14].
5. Hippo Signaling Pathway and Disease
a. Hippo Signaling Pathway and Cancer
Cancer is a group of diseases involving abnormal cell growth with the potential to invade or spread to other multiple body regions. Although the Hippo pathway was first discovered for its pivotal role in restricting imaginal disc growth by promoting cell-cycle exit and apoptosis, more recent studies in animal models have expanded the function of this pathway into other developmental contexts, such as papillary kidney cancers[28], colorectal cancer[29], ovarian cancer[30], breast cancer[31], and gastric cancer[32]. Carole Sourbier’s study revealed that targeted inhibition of Yes in YAP-activated tumors with dasatinib may present a therapeutic potential for patients with NF2-deficient PRCC tumors that have lost regulation by the Hippo signaling pathway[28]. Xu Wang’s research suggested that TFAP2C promotes CSCs characteristics and chemoresistance via transcriptionally activating negative regulators of Hippo signaling, ROCK1 and ROCK2, resulting in inactivation of Hippo signaling in colorectal cancer (CRC) cells[29]. The YAP/TAZ-TEAD transcription factor complex represents a universal target of oncogenic transformation. Up-regulation of the YAP gene locus has been reported at varying frequencies in a wide spectrum of human and murine tumors, such as medulloblastomas, carcinomas of the lung, pancreas, esophagus, liver, and mammary gland[33].
b. Hippo Signaling Pathway in Cardiovascular Development
Heart disease still is the main risk of death in both developed and developing countries. Heart malformation could lead to embryonic or postnatal death, and strenuous stimulations like pressure overload and/or ischemia could cause irreversible damage. Emerging evidences shows that the Hippo signaling pathway participates in cardiovascular development, hypertrophy, apoptosis, autophagy, angiogenesis, and regeneration[34][35][36][37][38].
Heallen reported that the Hippo signaling pathway is inactivated via cardiac-specific knockout of SAV1, which evidently decreases phosphorylated YAP level but not total YAP, resulting in an enlarged heart without alterations in cell size. Similar results were found in MST1/2 and LATS2 knockout mice[34]. D. P. Del Re et al. showed that treatment RASSF1A transgenic (TG) mice with adenoviral system increases MST1 phosphorylation and promotes cardiomyocyte apoptosis. But the proliferation ability of fibroblast and cardiac hypertrophy are reduced by exposing them to pressure overload[35]. Moreover, D. P. Del Re et al. another research showed that Cardiac-specific inactivation of YAP1 using α-MHC Cre recombinase transgenic mice Causes increased cardiomyocyte apoptosis in YAP(−/−) at baseline[36].
In this article, we introduce the relationship of the Hippo signaling pathway and other pathway and disease in brief, respectively. If you have any questions, you can leave a massage by clicking the “Contact Us” button in the bottom.
References
[1] Azucena Ramos, Fernando D. Camargo. The Hippo signaling pathway and stem cell biology[J]. Trends in Cell Biology. 2012, 22(7):339-346.
[2] Zhao B, Tumaneng K, et al. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat. Cell Biol. 2011, 13(8):877-83.
[3] Yang W, Han W, et al. The emerging role of Hippo signaling pathway in regulating osteoclast formation[J]. J Cell Physiol. 2018, 233(6):4606-4617.
[4] Pan D. The hippo signaling pathway in development and cancer[J]. Dev Cell. 2010,19(4):491-505.
[5] Camargo FD. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr. Biol. 2007, 17:2054-2060.
[6] Dong J. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007, 130:1120-1133.
[7] Wanlei Yang, Weiqi Han, et al. The emerging role of Hippo signaling pathway in regulating osteoclast formation[J]. J Cell Physiol. 2018, 233(6):4606-4617.
[8] Song, H., Kim, H., et al. Ablation of Rassf2 induces bone defects and subsequent haematopoietic anomalies in mice[J]. EMBO J. 2012, 31:1147-1159.
[9] Larsson, J., Ohishi, M., et al. Nf2/merlin regulates hematopoietic stem cell behavior by altering microenvironmental architecture[J]. Cell Stem Cell. 2008, 3: 221-227.
[10] Allen, N.P., Donninger, H., et al. RASSF6 is a novel member of the RASSF family of tumor suppressors[J]. Oncogene. 2007, 26: 6203-6211.
[11] Maruyama, R., Akino, K., et al. Cytoplasmic RASSF2A is a proapoptotic mediator whose expression is epigenetically silenced in gastric cancer[J]. Carcinogenesis. 2008, 29: 1312-1318.
[12] Lee, J.K., Shin, J.H., et al. MST1 functions as a key modulator of neurodegeneration in a mouse model of ALS[J]. Proc Natl Acad Sci USA. 2013, 110:12066-12071.
[13] Wang, S., Ma, K., et al. TAZ promotes cell growth and inhibits Celastrol-induced cell apoptosis[J]. Biosci Rep. 2016, 36.
[14] Carsten Gram Hansen, Toshiro Moroishi, et al. YAP and TAZ: a nexus for Hippo signaling and beyond[J]. Trends in Cell Biology. 2015, 1140, 1-15.
[15] Azzolin, L. Role of TAZ as mediator of Wnt signaling[J]. Cell, 2012, 151:1443-1456.
[16] Azzolin, L. YAP/TAZ incorporation in the b-catenin destruction complex orchestrates the Wnt response[J]. Cell, 2014, 158:157-170.
[17] Varelas, X. The Hippo pathway regulates Wnt/b-catenin signaling[J]. Dev. Cell. 2010, 18:579-591.
[18] Zhao, B. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control[J]. Genes Dev. 2007, 21:2747-2761.
[19] Takahashi, A. SHP2 tyrosine phosphatase converts parafibromin/Cdc73 from a tumor suppressor to an oncogenic driver[J]. Mol. Cell. 2011, 43:45-56.
[20] Varelas, X. The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease[J]. Development. 2014, 141:1614-1626.
[21] Vassilev, A. TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm[J]. Genes Dev. 2001, 15:1229-1241.
[22] Mo, J.S. The Hippo signaling pathway in stem cell biology and cancer[J]. EMBO Rep. 2014, 15: 642-656.
[23] Massague, J. TGFb signalling in context[J]. Nat. Rev. Mol. Cell Biol. 2012, 13: 616-630.
[24] Lai, D. and Yang, X. BMP4 is a novel transcriptional target and mediator of mammary cell migration downstream of the Hippo pathway component TAZ[J]. Cell. Signal. 2013,25:1720–1728.
[25] Varelas, X. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal[J]. Nat. Cell Biol. 2008,10:837-848.
[26] Judson, R.N. The Hippo pathway member Yap plays a key role in influencing fate decisions in muscle satellite cells[J]. J. Cell Sci. 2012, 125:6009–6019.
[27] Diepenbruck, M. Tead2 expression levels control the subcellular distribution of Yap and Taz, zyxin expression and epithelial-mesenchymal transition[J]. J. Cell Sci. 2014, 127:1523-1536.
[28] Sourbier C, Liao PJ, et al. Targeting loss of the Hippo signaling pathway in NF2-deficient papillary kidney cancers[J]. Oncotarget. 2018, 9(12):10723-10733.
[29] Xu Wang, Di Sun, et al. TFAP2C promotes stemness and chemotherapeutic resistance in colorectal cancer via inactivating hippo signaling pathway[J]. J Exp Clin Cancer Res. 2018, 37: 27.
[30] Xu M, Xiao J, et al. miR-149-5p promotes chemotherapeutic resistance in ovarian cancer via the inactivation of the Hippo signaling pathway[J]. Int J Oncol. 2018, 52(3):815-827.
[31] Liu J, Li J, et al. Loss of DLG5 promotes breast cancer malignancy by inhibiting the Hippo signaling pathway[J]. Sci Rep. 2017, 7(7):42125.
[32] Deng J, Lei W, et al. Cullin 4A (CUL4A), a direct target of miR-9 and miR-137, promotes gastric cancer proliferation and invasion by regulating the Hippo signaling pathway[J]. Oncotarget. 2016, 7(9):10037-50.
[33] Duojia Pan. The Hippo Signaling Pathway in Development and Cancer[J]. Developmental Cell. 2010, 10(19):401-505.
[34] T. Heallen, M. Zhang, et al. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size[J]. Science. 2011, 332: 458-461.
[35] D. P. Del Re, T. Matsuda, et al. Proapoptotic Rassf1A/ Mst1 signaling in cardiac fibroblasts is protective against pressure overload in mice[J]. The Journal of Clinical Investigation. 2010, 120(10): 3555-3567.
[36] D. P. Del Re, Y. Yang, et al. Yes-associated protein isoform 1 (Yap1) promotes cardiomyocyte survival and growth to protect against myocardial ischemic injury[J]. The Journal of Biological Chemistry. 2013, 288(6):3977-3988.
[37] G. Tao, P. C. Kahr, et al. Pitx2 promotes heart repair by activating the antioxidant response after cardiac injury[J]. Nature. 2016, 534:119-123.
[38] C. Joffre, N. Dupont, et al. The pro-apoptotic STK38 kinase is a new Beclin1 partner positively regulating autophagy[J]. Current Biology. 2015, 25(19):2479-2492.
CUSABIO team. The Overview of Hippo Signaling Pathway. https://www.cusabio.com/c-20633.html






Comments
Leave a Comment