|
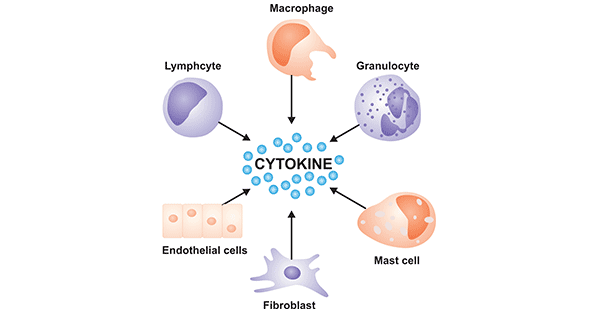
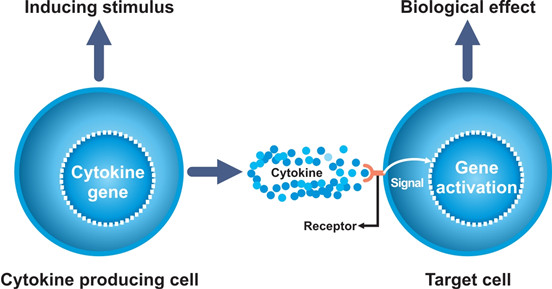
Detection of Cytokines
|
Advantages
|
Disadvantages
|
|
qPCR
|
High sensitivity, direct, quantitative, reproducible, easy and reliable data processing, allowing detection of many different cytokines from relatively small sample volumes.
|
Detection of relatively few samples, the presence of RNA does not always accurately reflect protein levels (like IL4, IL10 regulates post-translation), and the cell source that recognizes cytokines needs to separate different types of cells. This may be difficult, and if only a small fraction of the cells in the sample to be tested produce cytokines of interest, the threshold for detection may not be reached.
|
|
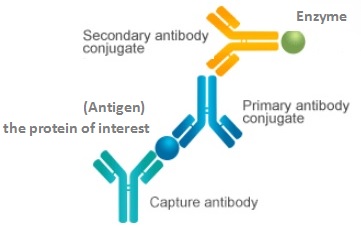
ELISA
|
Direct, quantitative
|
Smaller measurements (not always easy to get a sufficient amount of tissue fluid, and actual cytokine levels may be underestimated due to cytokine consumption); It is not possible to detect multiple cytokines in a single assay.
|
|
ELISPOT
|
Low-frequency cells that produce cytokines can be quickly identified and classified.
|
the same with ELISA
|
|
CBA
|
Use a small sample size to detect whole cytokine panels in multiple ways; Analysis is faster and adapts to high-throughput analysis.
|
High cost, low sensitivity, (as with ELISA) may underestimate cytokine production due to consumption.
|
|
IHC
|
Ability to identify cell types that produce cytokines (these cells may not be found by other methods): specificity, sensitivity, simplicity, and cost.
|
Poor quantitative, low sensitivity to detection of secreted proteins.
|
This article describes five methods for detecting cytokines in combination with the characteristics and functions of cytokines. Each method has its own advantages, but there are inevitably certain defects. Therefore, if you want to accurately measure the cytokine of a sample, you can combine two or more methods, because they can be compensated for each other.
In practical applications, they can be selected according to their respective experimental purposes and laboratory conditions. But no matter what method you consider to measure, the choice is based on the characteristics of the cytokine of interest.
Reference:
[1] Alick Isaacs, Jean Lindenmann. Virus interference. I. The interferon [J]. Proc. R. Soc. Lond. B Biol. Sci. 1957, 147 (927): 258–67.
[2] Finter NB, Chapman S, et al. The use of interferon-alpha in virus infections [J]. Drugs. 1991, 42(5): 749-65.
[3] Oppenheim, JJ Cytokines: past, present, and future [J]. Int. J. Hematol. 2001, 74:3-8.
[4] Dinarello CA. Proinflammatory cytokines [J]. Chest. 2000, 118: 503–8.
[5] Balkwill F, Charles KA, et al. Smoldering and polarized inflammation in the initiation and promotion of malignant disease [J]. Cancer Cell. 2005, 7:211–217.
[6] Eichten A, Coussens LM, et al. Paradoxical roles of the immune system during cancer development [J]. Nat Rev Cancer. 2006, 6:24–37.
[7] Bienvenu J, Monneret G, et al. The clinical usefulness of the measurement of cytokines. Clinical Chemistry and Laboratory Medicine. 2000; 38: 267–85.
[8] Woodman RC, Erickson RW, et al. Prolonged recombinant interferon-gamma therapy in chronic granulomatous disease: evidence against enhanced neutrophil oxidase activity [J]. Blood. 1992, 79 (6): 1558–62.
[9] Key LL, Rodriguiz RM, et al. Long-term treatment of osteopetrosis with recombinant human interferon gamma [J]. N. Engl. J. Med. 1995, 332 (24): 1594–9.
[10] Finter NB, Chapman S, et al. The use of interferon-alpha in virus infections [J]. Drugs. 1991, 42 (5): 749-65.
[11] Hoofnagle JH, Seeff LB. Peginterferon and ribavirin for chronic hepatitis C [J]. N Engl J Med. 2006, 355: 2444–51.
[12] Denuas L, Alcantud V, et al. Use of interferon-alpha in laryngeal papillomatosis: eight years of a Cuban national programme [J]. J Laryngol Otol. 1997, 111: 134–40.
[13] Jacobs L, O'Malley J, et al. Intrathecal interferon in multiple sclerosis [J]. Arch Neurol. 1982, 39:609–15.
[14] Rosenberg SA, JC Yang, et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2 [J]. JAMA. 1994, 271: 907–913.
[15] Rosenberg SA, JC Yang, et al. Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2: identification of the antigens mediating response [J]. Ann. Surg. 1998, 228: 307–319.
[16] Yang JC, RM Sherry, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer [J]. J. Clin. Oncol. 2003, 21: 3127–3132.
[17] Marcus JS, Anderson WF, et al. Microfluidic Single-Cell mRNA Isolation and Analysis [J]. Anal Chem. 2006, 78:3084–9.
[18] Marcus JS, Anderson WF, et al. Parallel Picoliter RT-PCR Assays Using Microfluidics [J]. Anal Chem. 2006;78:956–8.
[19] Diercks A, Kostner H, et al. Resolving cell population heterogeneity: real-time PCR for simultaneous multiplexed gene detection in multiple single-cell samples [J]. PLoS One. 2009, 4 No pp. given.
[20] Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays [J]. J Mol Endocrinol. 2000, 25:169-193.
[21] Allan SM, Harrison DC, et al. Selective increases in cytokine expression in the rat brain in response to striatal injection of a-amino-3-hydroxy-5-methyl-4-isoxazolepropionate and interleukin-1 [J]. Molecul Brain Res. 2001, 93:180-189.
[22] Favre N, Bordmann G, et al. Comparison of cytokine measurements using ELISA, ELISPOT and semi-quantitative RT-PCR [J]. J Immunol Methods. 1997, 204: 57–66.
[23] Ekerfelt C, Ernerudh J, et al. Detection of spontaneous and antigen-induced human interleukin-4 responses in vitro: comparison of ELISPOT, a novel ELISA and real-time RT-PCR [J]. J Immunol Methods. 2002, 260: 55–67.
[24] Atochina O, Mylvaganam R, et al. Comparison of results using the gel microdrop cytokine secretion assay with ELISPOT and intracellular cytokine staining assay [J]. Cytokine. 2004, 27:120–8.
[25] Kalyuzhny AE. Methods in Molecular Biology. Totowa, NJ: Humana Press; 2012. Handbook of ELISPOT: Methods and Protocols.
[26] Strand, Nacka. Find 1 cell in 100,000 with ELISpot. MABTECH. Retrieved 6 December 2018.
[27] Elshal MF and McCoy JP. Multiplex Bead Array Assays: Performance Evaluation and Comparison of Sensitivity to ELISA [J]. Methods. 2006, 38: 317–23.
[28] Carson RT, Vignali DAA. Simultaneous quantitation of 15 cytokines using a multiplexed flow cytometric assay [J]. J Immunol Methods. 1999, 227:41–52.
[29] Earley MC, Vogt RF, et al. Report from a workshop on multianalyte microsphere assays [J]. Cytometry. 2002, 50:239–42.
[30] Vignali DA. Multiplexed particle-based flow cytometric assays. J Immunol Methods[J]. 2000, 243:243–55.
[31] Khan SS, Smith MS, et al. Multiplex bead array for detection of soluble cytokines: comparisons of sensitivity and quantitative values among kits from multiple manufacturers [J]. Cytometry, Part B. 2004, 61B: 35–9.
[32] Morgan E, Varro R, et al. Cytometric bead array: a multiplexed assay platform with applications in various areas of biology [J]. Clin Immunol. 2004, 110: 252–66.




Comments
Leave a Comment