[1] Ji R, Wu C, Yao J, et al. IGF2BP2-mediated m6A modification of CSF2 reprograms MSC to promote gastric cancer progression. Cell Death and Disease, 2023, 14:693.
[2] Li Y, Zhai P, Zheng Y, et al. Csf2 Attenuated Sepsis-Induced Acute Kidney Injury by Promoting Alternative Macrophage Transition. Frontiers in Immunology, 2020, 11:1415.
[3] Hamilton JA. GM-CSF: from growth factor to central mediator of tissue inflammation. Frontiers in Immunology, 2019, 10:2055.
[4] Kitamura T, Hayashida K, Sakamaki K, et al. Reconstitution of functional receptors for human granulocyte/macrophage colony-stimulating factor (GM-CSF): evidence that the protein encoded by the AIC2B cDNA is a subunit of the murine GM-CSF receptor. Proceedings of the National Academy of Sciences, 1991, 88:5082-5086.
[5] Metcalf D. The biology of granulocyte-macrophage colony-stimulating factor. Blood, 1985, 66:1229-1236.
[6] Shuai K, Liu B, Ziff S, et al. JAK2 is associated with the GM-CSF receptor and is tyrosine phosphorylated and activated following receptor ligation. Cell, 1992, 70:519-529.
[7] Spina D, Zingarelli B, Greter M, et al. GM-CSF controls nonlymphoid tissue dendritic cell homeostasis but is dispensable for the differentiation of inflammatory dendritic cells. Immunity, 2012, 36:1031-1046.
[8] Hamilton JA. GM-CSF: from growth factor to central mediator of tissue inflammation. Frontiers in Immunology, 2019, 10:2055.
[9] Zingarelli B, Helft J, Chow A, et al. GM-CSF controls nonlymphoid tissue dendritic cell homeostasis but is dispensable for the differentiation of inflammatory dendritic cells. Immunity, 2012, 36:1031-1046.
[10] Rousselle A, Sonnemann J, Amann K, et al. CSF2-dependent monocyte education in the pathogenesis of ANCA-induced glomerulonephritis. Annals of the Rheumatic Diseases, 2022, 81:1162-1172.
[11] Ji R, Wu C, Yao J, et al. IGF2BP2-mediated m6A modification of CSF2 reprograms MSC to promote gastric cancer progression. Cell Death and Disease, 2023, 14:693.
[12] Huen SC, Huynh L, Marlier A, et al. GM-CSF promotes macrophage alternative activation after renal ischemia/reperfusion injury. Journal of the American Society of Nephrology, 2015, 26:1334-1345.
[13] Li Y, Zhai P, Zheng Y, et al. Csf2 Attenuated Sepsis-Induced Acute Kidney Injury by Promoting Alternative Macrophage Transition. Frontiers in Immunology, 2020, 11:1415.
[14] Shuai K, Liu B, Ziff S, et al. JAK2 is associated with the GM-CSF receptor and is tyrosine phosphorylated and activated following receptor ligation. Cell, 1992, 70:519-529.
[15] Spina D, Zingarelli B, Greter M, et al. GM-CSF controls nonlymphoid tissue dendritic cell homeostasis but is dispensable for the differentiation of inflammatory dendritic cells. Immunity, 2012, 36:1031-1046.
[16] Li Y, Zhai P, Zheng Y, et al. Csf2 Attenuated Sepsis-Induced Acute Kidney Injury by Promoting Alternative Macrophage Transition. Frontiers in Immunology, 2020, 11:1415.
[17] Rousselle A, Sonnemann J, Amann K, et al. CSF2-dependent monocyte education in the pathogenesis of ANCA-induced glomerulonephritis. Annals of the Rheumatic Diseases, 2022, 81:1162-1172.
[18] Ji R, Wu C, Yao J, et al. IGF2BP2-mediated m6A modification of CSF2 reprograms MSC to promote gastric cancer progression. Cell Death and Disease, 2023, 14:693.
[19] Hamilton JA. GM-CSF: from growth factor to central mediator of tissue inflammation. Frontiers in Immunology, 2019, 10:2055.
[20] Shuai K, Liu B, Ziff S, et al. JAK2 is associated with the GM-CSF receptor and is tyrosine phosphorylated and activated following receptor ligation. Cell, 1992, 70:519-529.
[21] Spina D, Zingarelli B, Greter M, et al. GM-CSF controls nonlymphoid tissue dendritic cell homeostasis but is dispensable for the differentiation of inflammatory dendritic cells. Immunity, 2012, 36:1031-1046.
[22] Li Y, Zhai P, Zheng Y, et al. Csf2 Attenuated Sepsis-Induced Acute Kidney Injury by Promoting Alternative Macrophage Transition. Frontiers in Immunology, 2020, 11:1415.
[23] Ji R, Wu C, Yao J, et al. IGF2BP2-mediated m6A modification of CSF2 reprograms MSC to promote gastric cancer progression. Cell Death and Disease, 2023, 14:693.
[24] Helft J, Bottcher J, Chakravarty P, et al. GM-CSF mouse bone marrow cultures comprise a heterogeneous population of Cd11c(+)Mhcii(+) macrophages and dendritic cells. Immunity, 2015, 42:1197-1211.
[25] Huen SC, Huynh L, Marlier A, et al. GM-CSF promotes macrophage alternative activation after renal ischemia/reperfusion injury. Journal of the American Society of Nephrology, 2015, 26:1334-1345.
[26] Ji R, Wu C, Yao J, et al. IGF2BP2-mediated m6A modification of CSF2 reprograms MSC to promote gastric cancer progression. Cell Death and Disease, 2023, 14:693.
[27] Liao R, Chen X, Cao Q, et al. HIST1H1B Promotes Basal-Like Breast Cancer Progression by Modulating CSF2 Expression. Frontiers in Oncology, 2021, 11:780094.
[28] Hamilton JA. GM-CSF: from growth factor to central mediator of tissue inflammation. Frontiers in Immunology, 2019, 10:2055.
[29] Meisel C, Schefold JC, Pschowski R, et al. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. American Journal of Respiratory and Critical Care Medicine, 2009, 180:640-648.
[30] Huen SC, Huynh L, Marlier A, et al. GM-CSF promotes macrophage alternative activation after renal ischemia/reperfusion injury. Journal of the American Society of Nephrology, 2015, 26:1334-1345.
[31] Ji R, Wu C, Yao J, et al. IGF2BP2-mediated m6A modification of CSF2 reprograms MSC to promote gastric cancer progression. Cell Death and Disease, 2023, 14:693.
[32] Helft J, Bottcher J, Chakravarty P, et al. GM-CSF mouse bone marrow cultures comprise a heterogeneous population of Cd11c(+)Mhcii(+) macrophages and dendritic cells. Immunity, 2015, 42:1197-1211.
[33] Huen SC, Huynh L, Marlier A, et al. GM-CSF promotes macrophage alternative activation after renal ischemia/reperfusion injury. Journal of the American Society of Nephrology, 2015, 26:1334-1345.

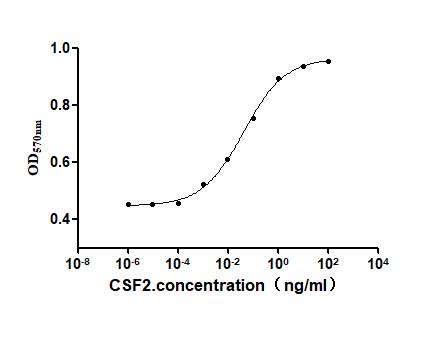
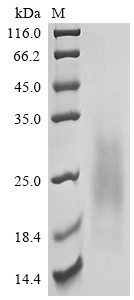
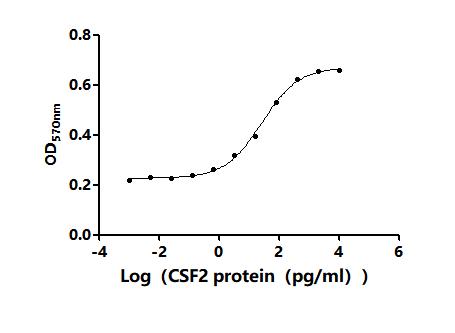
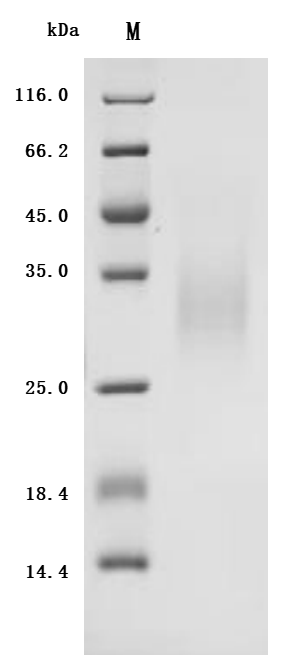
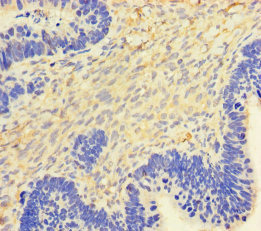
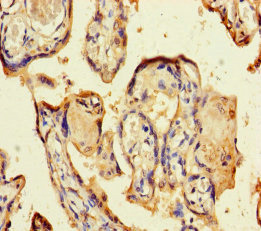
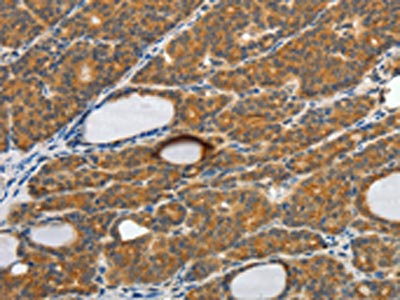

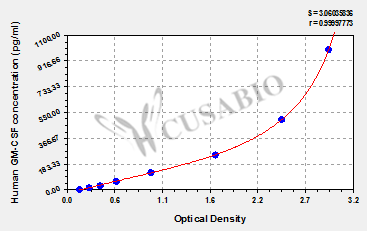



Comments
Leave a Comment