[1] Gruet, M., Cotton, D., Coveney, C., Boocock, D., Wagner, S., Komorowski, L., Rees, R., Pockley, A., Garner, A., Wallis, J., Miles, A., Powe, D., & Powe, D. (2020). β2-Adrenergic Signalling Promotes Cell Migration by Upregulating Expression of the Metastasis-Associated Molecule LYPD3. Biology, 9.
[2] Ying Huang et al. "Elevated Expression of LYPD3 Is Associated with Lung Adenocarcinoma Carcinogenesis and Poor Prognosis.." DNA and cell biology (2020).
[3] Yufei Hua et al. "m6A-dependent mature miR-151-5p accelerates the malignant process of HNSCC by targeting LYPD3." Molecular Biomedicine, 5 (2024).
[4] Yan Zhang et al. "Long non-coding RNA OGFRP1 regulates LYPD3 expression by sponging miR-124-3p and promotes non-small cell lung cancer progression.." Biochemical and biophysical research communications, 505 2 (2018): 578-585.
[5] Yuan-Jie Liu et al. "LY6/PLAUR domain containing 3 (LYPD3) maintains melanoma cell stemness and mediates an immunosuppressive microenvironment." Biology Direct, 18 (2023).
[6] T. Kanaseki et al. "LY6/PLAUR domain containing 3 has a role in the maintenance of colorectal cancer stem-like cells.." Biochemical and biophysical research communications, 486 2 (2017): 232-238.
[7] Tingting Hu et al. "LYPD3, a New Biomarker and Therapeutic Target for Acute Myelogenous Leukemia." Frontiers in Genetics, 13 (2022).
[8] Xinyao Xu et al. "Comprehensive analysis of exosome gene LYPD3 and prognosis/immune cell infiltration in lung cancer." Translational Cancer Research, 13 (2023): 1394 - 1405.

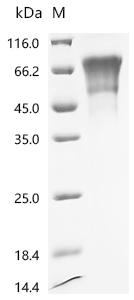
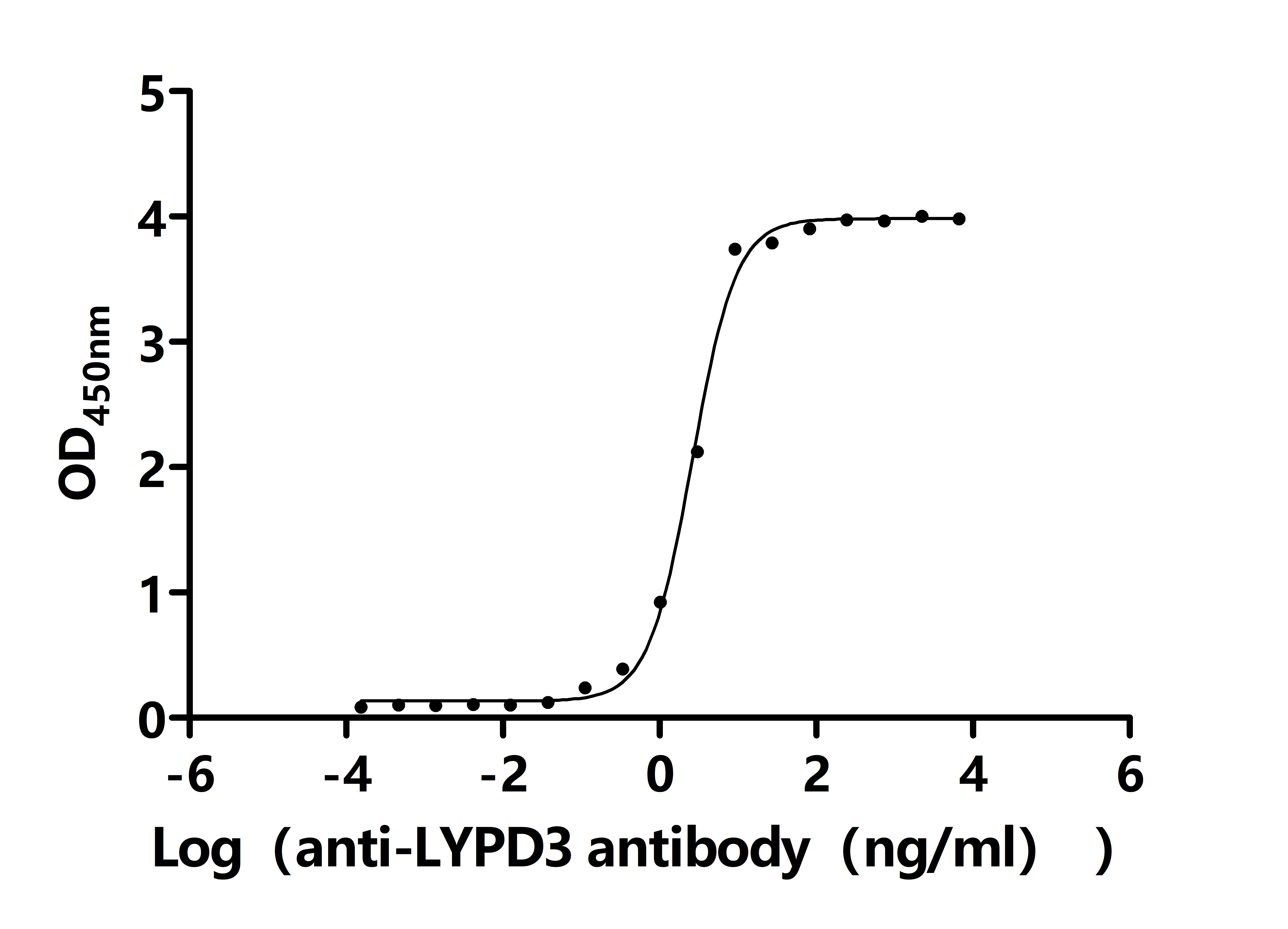
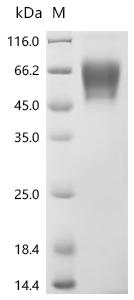
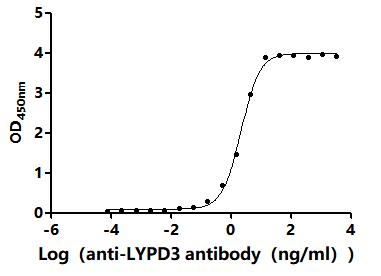
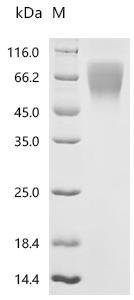
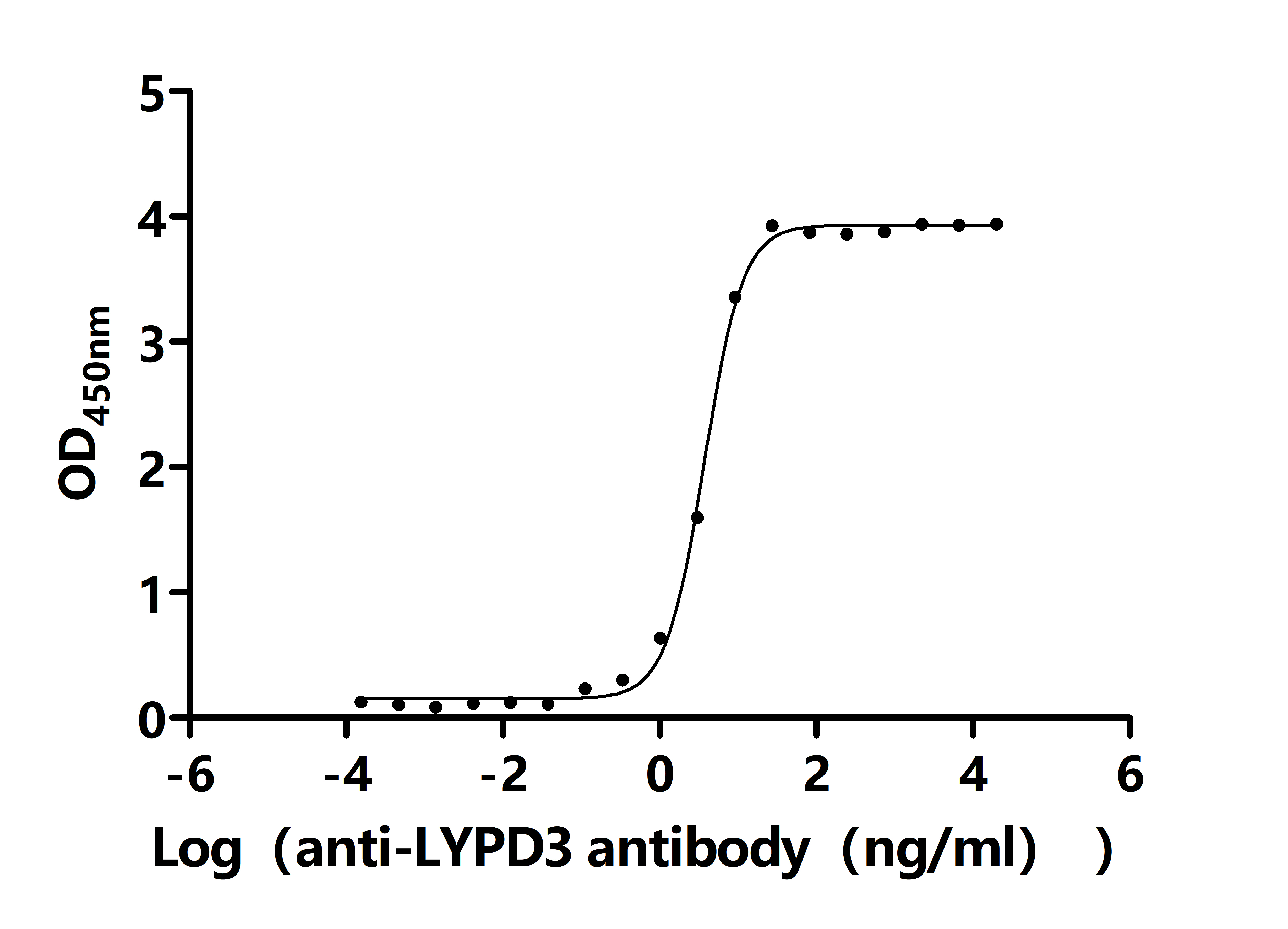
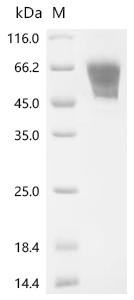
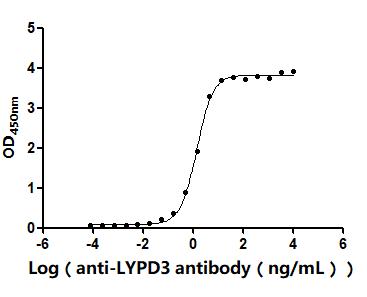



Comments
Leave a Comment