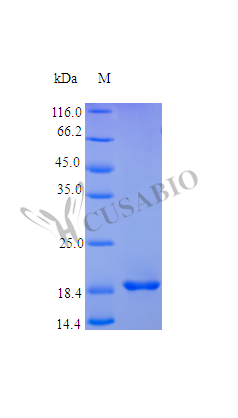
Recombinant Human Fibroblast Growth Factor 4 (FGF4) is expressed in E. coli and covers amino acids 25-206, maintaining a high purity level of over 96% as confirmed by SDS-PAGE analysis. This tag-free protein demonstrates full biological activity, with an ED50 of less than 0.5 ng/ml as measured by thymidine uptake assay in FGF-receptors transfected BaF3 cells, translating to a specific activity exceeding 2.0 × 10^6 IU/mg. The endotoxin level is maintained below 1.0 EU/µg according to the LAL method.
Fibroblast Growth Factor 4 (FGF4) appears to be one of the more critical proteins when it comes to cell proliferation, differentiation, and development. As part of the fibroblast growth factor family, it likely plays vital roles in embryonic development and tissue repair processes. FGF4 is known to activate several signaling pathways that seem essential in various biological processes. This makes it a significant focus of study in developmental biology and regenerative medicine research, though our understanding of its complete mechanisms continues to evolve.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
1. Cell Proliferation and Growth Factor Signaling Studies
This recombinant FGF4 is highly biologically active (ED₅₀ < 0.5 ng/ml) and suitable for proliferation and signaling studies. However, the partial sequence may alter signaling kinetics or amplitude compared to full-length FGF4. Researchers should validate that key signaling pathways (MAPK, PI3K) activation matches full-length FGF4, particularly regarding sustained versus transient signaling patterns. The high potency supports reliable dose-response studies, but optimal concentrations may need adjustment for primary cells versus transfected cell lines.
2. FGF Receptor Binding and Interaction Studies
The protein is appropriate for FGFR binding studies, but the partial sequence may affect heparin-dependent receptor dimerization - a critical mechanism for FGF signaling. Binding assays should include heparin supplementation and compare results with full-length FGF4 to confirm native-like binding characteristics. The tag-free design ensures accurate measurements, but the missing N-terminal region may influence receptor specificity profiles.
3. Antibody Development and Validation
This high-purity FGF4 serves as a good immunogen, but antibodies generated against this partial sequence will not recognize epitopes in the missing N-terminal region. Comprehensive antibody validation should include testing against full-length FGF4 to ensure recognition of all functional domains. The confirmed high bioactivity indicates proper folding of core epitopes.
4. Biochemical and Structural Analysis
The protein is suitable for structural studies, but the partial sequence lacks the complete heparin-binding domain present in full-length FGF4. Biophysical characterization should account for potential differences in stability and heparin-binding affinity compared to the native protein. Structural conclusions should be contextualized as representing a partially truncated form of FGF4.
5. Cell Culture Supplementation for Research Applications
This FGF4 can be used as a culture supplement, but the partial sequence may have different stability and half-life in culture conditions compared to full-length FGF4. Researchers should validate that proliferation effects match those of full-length FGF4, particularly for long-term culture applications where protein stability is crucial.
Final Recommendation & Action Plan
This recombinant human FGF4 partial protein (25-206aa) demonstrates exceptional biological activity, making it suitable for most proposed applications with appropriate validation of its partial nature. Prioritize confirming that key FGF4 functions (particularly heparin-dependent receptor dimerization and signaling duration) match full-length FGF4 in your experimental systems. For cell-based assays, the extremely high potency allows for low working concentrations, but includes heparin supplementation to ensure proper receptor activation. When developing antibodies, supplement with the full-length antigen to ensure comprehensive epitope coverage. For structural studies, the protein provides a valuable tool for analyzing the core FGF domain, but acknowledges the missing N-terminal region in interpretations. The E. coli expression produces a non-glycosylated protein, which is advantageous for FGF4 as it is typically non-glycosylated, but the partial sequence remains a significant consideration. Always validate critical findings with full-length FGF4 when studying complex biological contexts involving heparin interactions or receptor specificity.