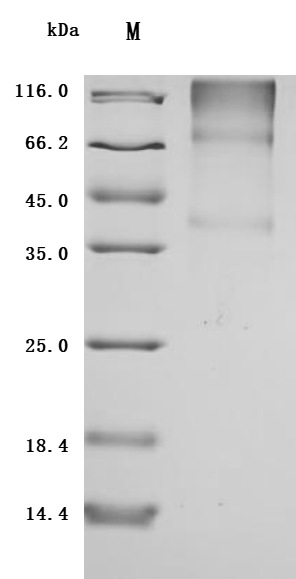
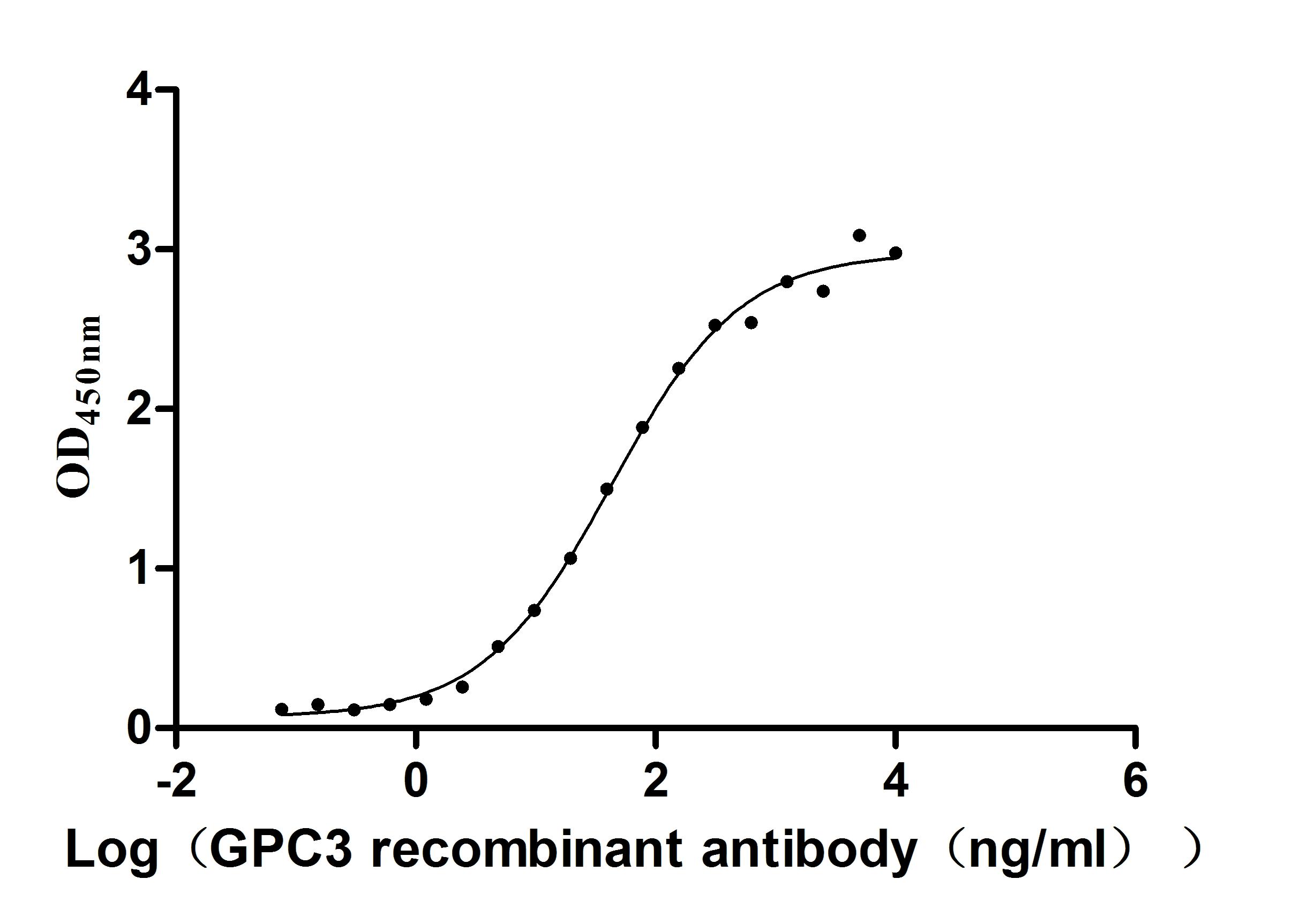
The recombinant human GPC3 protein is an active form produced in mammalian cells to ensure correct folding and native glycosylation, which are essential for its biological function. This construct includes amino acids 25 to 559 of human GPC3 protein and is tagged at the C-terminus with a 6xHis sequence to facilitate purification and detection. Supplied as a lyophilized powder, the recombinant GPC3 protein maintains high purity, exceeding 95% as verified by SDS-PAGE, and has an endotoxin level of less than 1.0 EU/μg, as confirmed by LAL testing. Functional activity is demonstrated by ELISA, where immobilized GPC3 at 5 μg/mL binds specifically to the anti-GPC3 recombinant antibody, yielding an EC50 between 35.19 and 52.30 ng/mL. These features make the GPC3 protein suitable for use in immunoassays, antibody screening, and cancer research.
Human GPC3 is a heparan sulfate proteoglycan anchored to the cell membrane via glycosylphosphatidylinositol. It plays a vital role in cellular processes, including growth, differentiation, and invasion, especially within the context of various cancers, most notably hepatocellular carcinoma (HCC) [1][2][3]. This protein is encoded by the GPC3 gene located on the X chromosome and exhibits unique expression patterns in different tissues, being highly expressed in fetal tissues but typically absent in many adult tissues, rendering it an oncofetal marker [3][4].
GPC3 is particularly relevant in cancer research due to its overexpression in specific malignancies. For instance, it is significantly overexpressed in HCC, serving as a valuable biomarker for diagnosis and prognosis [1][2][5]. Its expression levels correlate with tumor progression and have been associated with poor clinical outcomes [6][7]. Studies have shown that GPC3 may modulate signaling pathways related to growth factors, such as fibroblast growth factor 2, which further highlights its role in tumor growth and development [7][8]. Moreover, soluble GPC3 can be detected in the sera of patients with HCC, making it a potential target for immunotherapy [9][10].
Additionally, GPC3 is linked to Simpson-Golabi-Behmel syndrome (SGBS), a genetic condition characterized by overgrowth and various physical anomalies. This relationship emphasizes GPC3's role in development as well as its potential implications in tumorigenesis [11][3]. While some research has suggested a tumor-suppressive role of GPC3 in certain contexts, its expression in cancer typically indicates a growth-promoting function [2][12].
References:
[1] X. Jia, J. Liu, Y. Gao, Y. Huang, & D. Zhi. Diagnosis accuracy of serum glypican-3 in patients with hepatocellular carcinoma: a systematic review with meta-analysis. Archives of Medical Research, vol. 45, no. 7, p. 580-588, 2014. https://doi.org/10.1016/j.arcmed.2014.11.002
[2] Y. Sung, S. Hwang, et al. Glypican‐3 is overexpressed in human hepatocellular carcinoma. Cancer Science, vol. 94, no. 3, p. 259-262, 2003. https://doi.org/10.1111/j.1349-7006.2003.tb01430.x
[3] B. Moudi, Z. Heidari, & H. Mahmoudzadeh‐Sagheb. Meta-analysis and systematic review of prognostic significance of glypican-3 in patients with hepatitis b-related hepatocellular carcinoma. Virusdisease, vol. 30, no. 2, p. 193-200, 2019. https://doi.org/10.1007/s13337-019-00517-6
[4] Y. Motomura, Y. Ikuta, et al. Hla-a2 and -a24-restricted glypican-3-derived peptide vaccine induces specific ctls: preclinical study using mice. International Journal of Oncology, 2008. https://doi.org/10.3892/ijo.32.5.985
[5] A. Attallah, M. El‐Far, et al. Gpc-hcc model: a combination of glybican-3 with other routine parameters improves the diagnostic efficacy in hepatocellular carcinoma. Tumor Biology, vol. 37, no. 9, p. 12571-12577, 2016. https://doi.org/10.1007/s13277-016-5127-6
[6] H. Shirakawa, H. Suzuki, et al. Glypican‐3 expression is correlated with poor prognosis in hepatocellular carcinoma. Cancer Science, vol. 100, no. 8, p. 1403-1407, 2009. https://doi.org/10.1111/j.1349-7006.2009.01206.x
[7] T. Ishiguro, M. Sugimoto, et al. Anti–glypican 3 antibody as a potential antitumor agent for human liver cancer. Cancer Research, vol. 68, no. 23, p. 9832-9838, 2008. https://doi.org/10.1158/0008-5472.can-08-1973
[8] D. Kandil, G. Leiman, M. Allegretta, & M. Evans. Glypican‐3 protein expression in primary and metastatic melanoma. Cancer Cytopathology, vol. 117, no. 4, p. 271-278, 2009. https://doi.org/10.1002/cncy.20032
[9] T. Hickman, E. Choi, et al. Boxr1030, an anti-gpc3 car with exogenous got2 expression, shows enhanced t cell metabolism and improved anti-cell line derived tumor xenograft activity. Plos One, vol. 17, no. 5, p. e0266980, 2022. https://doi.org/10.1371/journal.pone.0266980
[10] T. Nakatsura, T. Kageshita, et al. Identification of glypican-3 as a novel tumor marker for melanoma. Clinical Cancer Research, vol. 10, no. 19, p. 6612-6621, 2004. https://doi.org/10.1158/1078-0432.ccr-04-0348
[11] D. Cano-Gauci, H. Song, et al. Glypican-3–deficient mice exhibit developmental overgrowth and some of the abnormalities typical of simpson-golabi-behmel syndrome. The Journal of Cell Biology, vol. 146, no. 1, p. 255-264, 1999. https://doi.org/10.1083/jcb.146.1.255
[12] Y. Zhang, S. Lin, Z. Xiao, & M. Ho. A proteomic atlas of glypican‐3 interacting partners: identification of alpha‐fetoprotein and other extracellular proteins as potential immunotherapy targets in liver cancer. Proteoglycan Research, vol. 2, no. 4, 2024. https://doi.org/10.1002/pgr2.70004