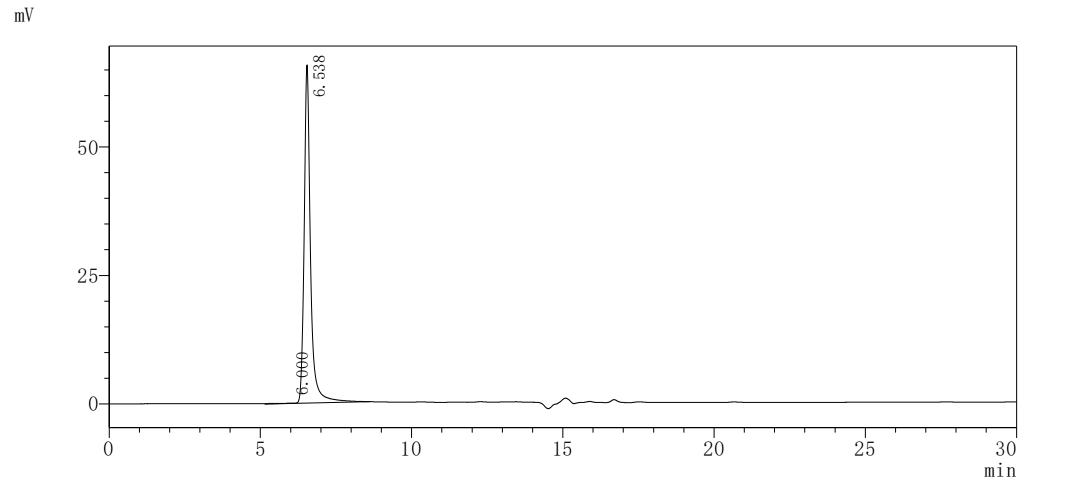
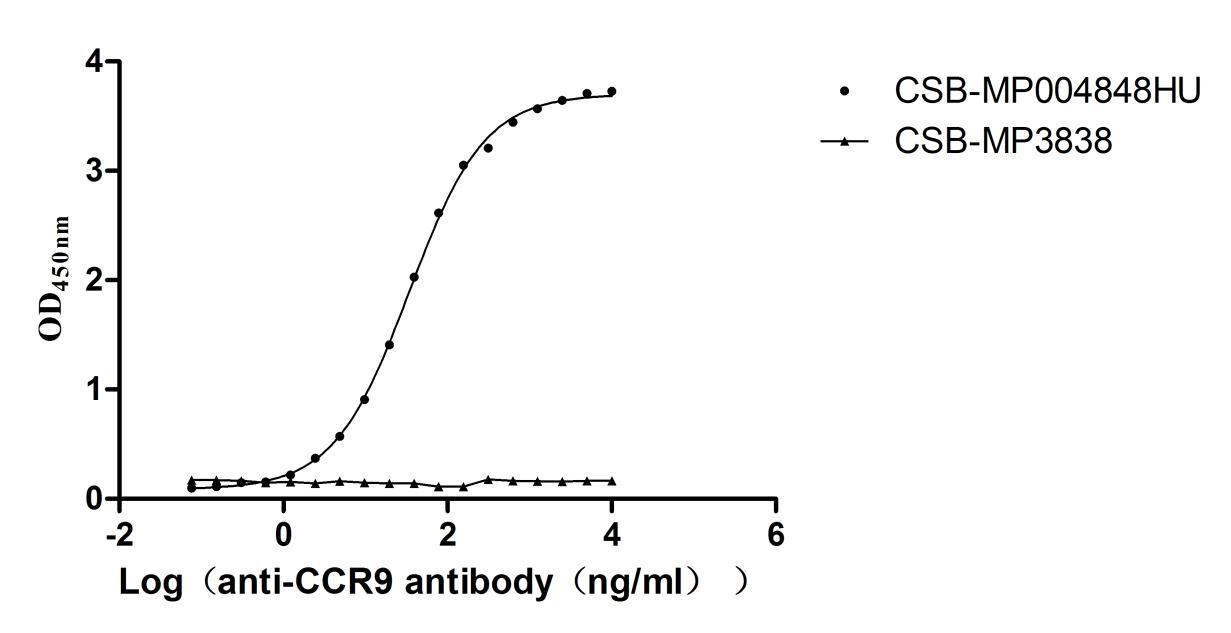
The recombinant human CCR9 protein is a mammalian cell-derived virus-like particle (VLP) formulation engineered to present the full-length human C-C chemokine receptor type 9, spanning residues 1-369, with a C-terminal 10xHis tag for purification. Produced as lyophilized powder, this VLP-embedded protein exhibits >95% purity as validated by SEC-HPLC, ensuring monodispersity and structural integrity. Functional characterization via ligand-binding assays demonstrates its capacity to interact specifically with the anti-CCR9 recombinant antibody (CSB-RA004848MA1HU) when immobilized at 10 μg/mL, yielding an effective concentration for half-maximal binding (EC50) between 31.67 and 36.83 ng/mL. The VLP architecture mimics native membrane topology, preserving conformational epitopes critical for studying receptor-ligand interactions and antibody neutralization mechanisms. This particulate presentation system enhances avidity effects while maintaining solubility, making it particularly suitable for vaccine development, immune response profiling, and high-throughput screening of therapeutic candidates targeting chemokine-mediated signaling pathways.
The CCR9 protein, a crucial member of the chemokine receptor family, has emerged as a significant player in the immune response and various cancers. CCR9's primary ligand, CCL25 (thymus-expressed chemokine), directs immune cell migration, particularly impacting T lymphocytes' localization to the intestines and thymus. This homing ability is vital for maintaining immune surveillance and tolerance in the gut, where CCR9+ T cells are preferentially recruited to sites of inflammation or injury, thus influencing local immune responses and potentially contributing to the pathogenesis of autoimmune diseases like Crohn's disease [1][2][3][4].
Functionally, CCR9 has been implicated in regulating T cell development and function. In particular, it is expressed on various T cell subsets, including CD4+ and CD8+ T cells, and plays a critical role in their differentiation and survival through signaling pathways such as PI3K/Akt [3][5]. CCR9+ dendritic cells also contribute to immune modulation, enhancing the immunological tolerance necessary to prevent inappropriate immune responses to commensal gut microbiota and food antigens [2][3].
The expression of CCR9 is not limited to the immune system. It has been shown to play a role in cancer pathology. Overexpression of CCR9 has been associated with increased cell motility and invasiveness in several malignancies, including lung adenocarcinoma, ovarian cancer, and melanoma [6][7][8]. CCR9 is essential for the normal immune function of lymphocytes homing to mucosal sites and also facilitates the metastasis of cancer cells to the intestinal environment. This property is particularly evident in studies demonstrating that CCR9+ tumor cells preferentially migrate to tissues where CCL25 is expressed, suggesting a mechanism for cancer progression and metastasis [7-9].
References:
[1] M. Pathak, P. Padghan, N. Halder, N. Kulkarni, S. Sonar, & G. Lal. Ccr9 signaling in dendritic cells drives the differentiation of foxp3+ tregs and suppresses the allergic ige response in the gut. European Journal of Immunology, vol. 50, no. 3, p. 404-417, 2019. https://doi.org/10.1002/eji.201948327
[2] H. Hadeiba, T. Sato, A. Habtezion, C. Oderup, J. Pan, & E. Butcher. Ccr9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft-versus-host disease. Nature Immunology, vol. 9, no. 11, p. 1253-1260, 2008. https://doi.org/10.1038/ni.1658
[3] M. Pathak and G. Lal. The regulatory function of ccr9+ dendritic cells in inflammation and autoimmunity. Frontiers in Immunology, vol. 11, 2020. https://doi.org/10.3389/fimmu.2020.536326
[4] L. Airaksinen, J. Cerqueira, et al. Dissecting the contribution of single nucleotide polymorphisms in ccr9 and ccl25 genomic regions to the celiac disease phenotype. Journal of Translational Autoimmunity, vol. 4, p. 100128, 2021. https://doi.org/10.1016/j.jtauto.2021.100128
[5] B. Li, Z. Wang, Y. Zhong, J. Lan, X. Li, & H. Lin. Ccr9–ccl25 interaction suppresses apoptosis of lung cancer cells by activating the pi3k/akt pathway. Medical Oncology, vol. 32, no. 3, 2015. https://doi.org/10.1007/s12032-015-0531-0
[6] Y. Zhong, L. Jiang, et al. Expression of cc chemokine receptor 9 predicts poor prognosis in patients with lung adenocarcinoma. Diagnostic Pathology, vol. 10, no. 1, 2015. https://doi.org/10.1186/s13000-015-0341-x
[7] E. Johnson, R. Singh, et al. Ccl25-ccr9 interaction modulates ovarian cancer cell migration, metalloproteinase expression, and invasion. World Journal of Surgical Oncology, vol. 8, no. 1, 2010. https://doi.org/10.1186/1477-7819-8-62
[8] Z. Zhang, C. Qin, Y. Wu, Z. Su, G. Xian, & B. Hu. Ccr9 as a prognostic marker and therapeutic target in hepatocellular carcinoma, Oncology Reports, vol. 31, no. 4, p. 1629-1636, 2014. https://doi.org/10.3892/or.2014.2998
[9] E. Heinrich, A. Arrington, et al. Paracrine activation of chemokine receptor ccr9 enhances the invasiveness of pancreatic cancer cells. Cancer Microenvironment, vol. 6, no. 3, p. 241-245, 2013. https://doi.org/10.1007/s12307-013-0130-6