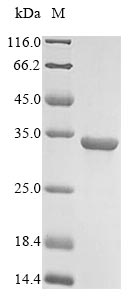
Recombinant Human Pro-glucagon (GCG) is produced in E. coli and comes with an N-terminal 6xHis-GST tag. This partial protein covers amino acids 53-89 and appears to be purified to greater than 85% purity based on SDS-PAGE analysis. The product is intended for research use only, though it seems to perform consistently across different experimental setups.
Pro-glucagon acts as a precursor to several key peptides, including glucagon and GLP-1. These peptides play important roles in glucose metabolism and insulin regulation. The derivatives from pro-glucagon are involved in pathways related to energy balance and have become a central focus in metabolic research and diabetes studies.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
The human pro-glucagon is a precursor protein that requires specific proteolytic processing to generate bioactive hormones (e.g., glucagon, GLP-1). The expressed fragment (53-89aa) is short (37 amino acids) and may include portions of glucagon (33-61aa) and GLP-1 (78-107aa) regions, but it lacks the full sequences needed for independent bioactivity (e.g., receptor binding). The large GST tag (∼26 kDa) may improve solubility but can sterically hinder the correct folding or function of the small fragment. E. coli expression often fails to replicate eukaryotic post-translational modifications or native conformational states. While small peptides may fold simply, the tag dominance and absence of contextual protein regions make correct folding unlikely. Bioactivity (e.g., hormonal function) is improbable, but the fragment could retain linear epitopes or protease cleavage sites. Without validation (e.g., circular dichroism for structure or activity assays), the protein is likely misfolded or inactive for native functions.
1. Antibody Development and Validation Studies
This recombinant pro-glucagon fragment can be used as an immunogen for generating antibodies targeting linear epitopes within amino acids 53-89. The His-GST tag simplifies purification and immobilization for ELISA-based screening. However, antibodies produced may not recognize native, correctly folded pro-glucagon or its processed hormones (e.g., in physiological samples), as conformational epitopes could be absent. Validation against full-length pro-glucagon or natural hormones from eukaryotic sources is essential for specificity in applications like Western blot or immunoassay.
2. Biochemical Characterization and Structural Studies
The protein is suitable for basic biophysical analyses (e.g., circular dichroism to assess secondary structure, dynamic light scattering to monitor aggregation). These studies can provide insights into the fragment’s stability and folding behavior under various conditions. However, due to the large tag and potential misfolding, it is not appropriate for high-resolution structural studies (e.g., X-ray crystallography) aimed at understanding native pro-glucagon structure.
3. Enzyme Substrate Studies
If the fragment contains known protease cleavage sites (e.g., for prohormone convertases within 53-89aa), it could be used as a substrate in vitro to study enzyme kinetics and cleavage specificity. The tags facilitate the detection of cleavage products via Western blot. However, misfolding might alter accessibility to cleavage sites, so results should be compared with native peptides or full-length pro-glucagon to confirm validity.
Final Recommendation & Action Plan
Before using this recombinant pro-glucagon fragment for functional applications, validate its folding and suitability for intended studies. Start with biophysical assays (e.g., circular dichroism to check for expected structural elements, such as α-helical content in glucagon-related regions) and functional tests (e.g., protease cleavage assays with positive controls). For antibody production, proceed but rigorously validate antibodies against natural pro-glucagon or hormones. Avoid interaction studies without confirmation of native-like folding. For reliable results on pro-glucagon biology, consider using longer constructs or eukaryotic-expressed proteins that better replicate physiological conditions.