Diabetes mellitus (DM) is a kind of clinical syndrome caused by insufficient insulin secretion or deficiency of insulin function. It mainly manifested as chronic hyperglycemia and is usually accompanied by a variety of cardiovascular diseases. It can lead to microvasculopathy (retinopathy and nephropathy), macrovascular (cardiovascular disease) and neurological diseases.
With the acceleration of modernization and the change of lifestyle, the prevalence of diabetes is on the rise globally, and the treatment of diabetes become a common problem all over the world.
1. Epidemiology of Diabetes
Diabetes is a global issue, no country is immune from diabetes and the epidemic is expected to continue. In this new edition of the IDF Diabetes Atlas, the prevalence of diabetes are estimated for the years 2017 and 2045.
The number of diabetics worldwide is estimated at 425 million in 2017 and is expected to reach 629 million by 2045. The global average prevalence of diabetes is 48 %. The estimates are provided for seven IDF regions: Africa (AFR), Europe (EUR), Middle East and North Africa (MENA), North America and Caribbean (NAC), South and Central America (SACA), South-East Asia (SEA) and the Western Pacific (WP).
1.1 Regional Distribution
Currently, the area with the highest prevalence is the western Pacific, but the growth rate is relatively low. In contrast, the prevalence of diabetes are low in Africa, with only 16 million people in 2017. But its growth rate is as high as 156 percent. The specific data is shown in figure 1.
1.2 Age Distribution
There are 326.5 million people 20-64 years with diabetes, and 122.8 million people 65-79 years with diabetes. Although the number of people with diabetes is higher in the 20-64 age groups than 65-79 years, the rate of increase is significantly faster for 65-79 years. The most important demographic change to diabetes prevalence across the world appears to be the increase in the proportion of people 65 years of age [1]. Given the increasing prevalence of obesity, these data may understate the prevalence of diabetes in the future.
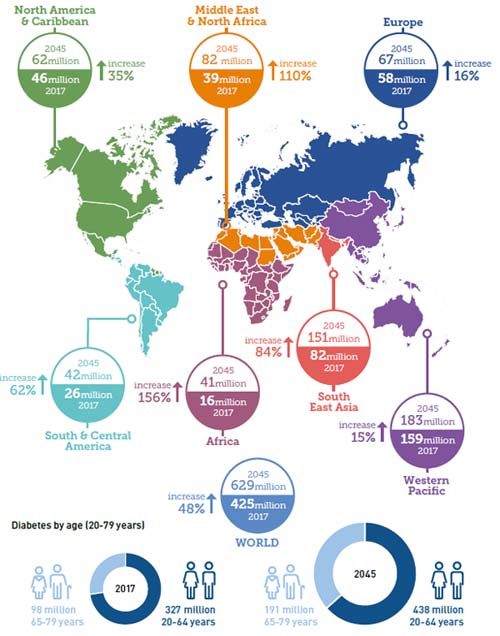
Figure1 Number of people with diabetes worldwide and per region in 2017 and 2045 (20-79 years)
*The sources of data and picture is derived from the international diabetes federation
2. Classification of Diabetes
In generally, Diabetes is divided into four types: type l (insulin dependent diabetes mellitus, IDDM), type 2 (non-insulin dependent diabetes mellitus, NIDDM), gestational diabetes mellitus and special type diabetes mellitus.
2.1 Type 1 Diabetes Mellitus
Type 1 diabetes mellitus (T1DM) also known as insulin dependent diabetes, is a disease of absolute insulin deficiency caused by the destruction of pancreatic cells[2] .Worldwide, the prevalence rate is increasing at an annual rate of 2 % to 3 % [3]. At a low age, the disease increases significantly[4][5]. People with T1DM accounts for only 5 % ~ 10 % of the global diabetes, and type 2 diabetes accounts for the vast majority.
2.2 Type 2 Diabetes Mellitus
Type 2 diabetes mellitus is an abnormal metabolism of carbohydrates, proteins, and fats resulting from insulin secretion deficiency or insulin deficiency. Type 2 diabetes is generally mild, hidden and has a long course. It is more common in adults, especially the elderly.
2.3 Gestational Diabetes Mellitus
Gestational diabetes mellitus (GDM) refers to women who have no diabetes and low glucose tolerance in the past. It is the first time that abnormal glucose tolerance is detected during pregnancy. Gestational diabetes can increase the morbidity and mortality of pregnant women and fetuses. The risk of obesity and diabetes in offspring is also significantly increased. The incidence of type 2 diabetes in patients with gestational diabetes during the first 20 years after delivery is up to 40 % [6].
2.4 Special Type Diabetes Mellitus
Special type diabetes mellitus mainly refers to some rare types of diabetes, for example, pancreatic diseases. This type of diabetes accounts for less than 1 % to 5 % of all diabetes cases.
3. Causes of Diabetes
At present, the cause of diabetes has not been fully elucidated. Type 2 diabetes mellitus (T2DM) has complicated causes. It is generally believed that its pathogenesis is closely related to genetic and environmental factors.
Genetic factors: The earlier studies found that the genes of glucose transporter protein-2, glucagon receptor gene (GCGR), hepatocyte nuclear factor-1α (HNF1A), -4α (HNF4A), -1β (HNF1B), glucokinase gene (GCK) and insulin promoter factor-1α are correlated with the abnormal secretion of insulin.
Environmental factors: Central obesity is the most important environmental factor causing T2DM. Lifestyle and age are also related factors affecting type 2 diabetes (older people are more likely to develop DM), one possible reason is that the ratio of muscle to fat in the body changes as your age and reduce physical activity. Chronic sleep deprivation can promote the development of T2DM, and mental factors are also important factors influencing type 2 diabetes. In addition, the incidence of type 2 diabetes is closely related to infection of some viral infections (such as intestinal virus, coxsackie virus, mumps virus, rubella virus, etc.). Oxidative stress, apoptosis of the oocytes, and inflammatory factors are also the causes of type 2 diabetes. The main reason is that the state of high-sugar and high-fat increases the reactive oxygen species (ROS) [7], weakens the antioxidant capacity of the body and causes a series of pathological changes. These abnormal conditions cause apoptosis of the insulin β cells.
Similar to type 2 diabetes, type 1 diabetes is also linked to heredity, however, more than 85 percent of patients whose first-degree relatives did not have the disease. Human leukocyte antigen (HLA) genes related to type 1 diabetes. In addition, virus infection and chemicals also have certain influence to type 1 diabetes. Therefore, the intensive study of gene-gene, gene-environment and epigenetic inheritance will provide evidence for the early diagnosis and prevention of type 1 diabetes [8].
In addition to the above factors, type 1 diabetes is associated with insulin resistance. Type 1 diabetes mostly occurs in children or adolescents. It is the main type of diabetes in children and adolescents. Type 1 diabetes is widely distributed in different regions, the prevalence in different regions ranges from high to low: Europe, North America, Australia, South America and Central America, Africa, Asia [9]. Vitamin D, temperature, and population density may affect the regional distribution of type 1 diabetes.
4. Signal Pathway
Recent studies have shown that protein kinase C (PKC) pathways, oxidative stress, growth factors, osmotic pressure and stretch stimulation in diabetes can all activate the MAPK family, increase the activity of transcription factors and participate in the development of chronic complications of diabetes. P38 mitogen-activated protein kinase (MAPK) is an important member of the MAPKs superfamily[10]. It is the junction of cell signaling pathways and plays an important role in cell metabolism, proliferation, differentiation and apoptosis.
In the diabetic nephropathies (DN), p38 MAPK can be activated by a variety of factors and then promote cell proliferation and extracellular matrix synthesis, increase production of class reactive oxygen species (ROS). Osman et al [11] found that activation of p38 MAPK can increase ROS production and promote the development of DN. This signal can also promote the formation of osteopontin (OPN) [12]and promote the schizolysis and shedding of Kidney injury molecule-1 (KIM-1). MAPK is associated with diabetic vascular complications and diabetic neuropathy. With the further study of the relationship between MAPK family and diabetic complications, blocking MAPK signaling pathways may become a new strategy for the treatment of diabetic complications. MAPK may become a new target for the treatment of chronic complications of diabetes.
5. Important proteins in Diabetes Research
Research on diabetes involves various proteins as it is a complex metabolic disorder involving multiple biological processes. Here are some key proteins related to diabetes research and their functions:
Insulin: One of the core issues in diabetes is insulin abnormalities. Diabetic patients may have insufficient insulin secretion or insensitivity to it.
| Target |
Uniprot ID |
Product Name |
Source |
Code |
| Ins1 |
P01322 |
Recombinant Rat Insulin-1(Ins1),partial |
E.coli |
CSB-EP355622RA |
| Ins1 |
P01325 |
Recombinant Mouse Insulin-1(Ins1) |
E.coli |
CSB-EP355623MO |
| Ins2 |
P01326 |
Recombinant Mouse Insulin-2(Ins2) |
E.coli |
CSB-EP360644MO |
| Ins1 |
P01325 |
Recombinant Mouse Insulin-1(Ins1) |
Yeast |
CSB-YP355623MO |
| INS |
P01311 |
Recombinant Rabbit Insulin(INS),partial |
E.coli |
CSB-EP011742RB |
| INS |
P01310 |
Recombinant Horse Insulin(INS),partial |
E.coli |
CSB-EP011742HO |
| INS |
P01317 |
Recombinant Bovine Insulin(INS),partial |
E.coli |
CSB-EP011742BO |
Diabetes-Related Genes: Including TCF7L2, PPARG, ADIPOQ, CAPN10, these genes are associated with glucose metabolism, insulin sensitivity, and insulin secretion.
| Target |
Uniprot ID |
Product Name |
Source |
Code |
| ADIPOQ |
Q3Y5Z3 |
Recombinant Bovine Adiponectin(ADIPOQ) |
E.coli |
CSB-EP661101BO |
| Adipoq |
Q60994 |
Recombinant Mouse Adiponectin(Adipoq) |
E.coli |
CSB-EP723362MO |
| Adipoq |
Q60994 |
Recombinant Mouse Adiponectin(Adipoq),partial |
E.coli |
CSB-RP079474m |
| ADIPOQ |
Q3Y5Z3 |
Recombinant Bovine Adiponectin(ADIPOQ) |
Yeast |
CSB-YP661101BO |
| ADIPOQ |
F7DZE7 |
Recombinant Horse Adiponectin D(ADIPOQ),partial |
E.coli |
CSB-EP4093HO |
| PPARG |
P37231 |
Recombinant Human Peroxisome proliferator-activated receptor gamma(PPARG) |
E.coli |
CSB-EP018424HU |
| Pparg |
P37238 |
Recombinant Mouse Peroxisome proliferator-activated receptor gamma(Pparg) |
E.coli |
CSB-EP018424MO |
| Pparg |
P37238 |
Recombinant Mouse Peroxisome proliferator-activated receptor gamma(Pparg) |
E.coli |
CSB-EP018424MOb0 |
| PPARG |
P37231 |
Recombinant Human Peroxisome prolifeRator-activated receptor gamma(PPARG) |
Yeast |
CSB-YP018424HU |
| pparg |
P37234 |
Recombinant Xenopus laevis Peroxisome proliferator-activated receptor gamma(pparg) |
E.coli |
CSB-EP018424XBE |
| TCF7L2 |
Q9NQB0 |
Recombinant Human Transcription factor 7-like 2(TCF7L2),partial |
E.coli |
CSB-EP889079HU |
Diabetes-Related Hormones: Such as Glucagon and Leptin, influencing blood glucose levels.
| Target |
Uniprot ID |
Product Name |
Source |
Code |
| LEP |
P41159 |
Recombinant Human Leptin(LEP),partial |
E.coli |
CSB-RP067974h |
| LEP |
P41159 |
Recombinant Human Leptin protein(LEP) (Active) |
E.coli |
CSB-AP000061HU |
GLUT (Glucose Transporter Proteins): These proteins mediate the entry of glucose into cell membranes, with GLUT4 playing a crucial role in muscle and adipose tissue.
Glycogen Synthase: Controls glycogen synthesis, essential for regulating blood sugar.
| Target |
Uniprot ID |
Product Name |
Source |
Code |
| GSK3B |
P49841 |
Recombinant Human Glycogen synthase kinase-3 beta(GSK3B) |
E.coli |
CSB-EP009963HU |
| GSK3B |
P49841 |
Recombinant Human Glycogen synthase kinase-3 beta(GSK3B) |
Yeast |
CSB-YP009963HU |
SGLT2 (Sodium-Glucose Cotransporter 2): This protein in the kidneys is related to drugs used in diabetes treatment.
PKC (Protein Kinase C): The PKC family plays a significant role in cell signaling under high glucose conditions.
AMPK (AMP-Activated Protein Kinase): Associated with energy metabolism and insulin sensitivity.
| Target |
Uniprot ID |
Product Name |
Source |
Code |
| Prkaa1 |
Q5EG47 |
Recombinant Mouse 5'-AMP-activated protein kinase catalytic subunit alpha-1(Prkaa1) |
Baculovirus |
CSB-BP707843MO |
| Prkaa1 |
Q5EG47 |
Recombinant Mouse 5-AMP-activated protein kinase catalytic subunit alpha-1(Prkaa1) |
Baculovirus |
CSB-BP707843MOa0 |
| PRKAB2 |
O43741 |
Recombinant Human 5'-AMP-activated protein kinase subunit beta-2(PRKAB2) |
E.coli |
CSB-EP527326HU |
| Prkag1 |
O54950 |
Recombinant Mouse 5-AMP-activated protein kinase subunit gamma-1(Prkag1) |
E.coli |
CSB-EP528783MO |
| Prkaa1 |
Q5EG47 |
Recombinant Mouse 5-AMP-activated protein kinase catalytic subunit alpha-1(Prkaa1),partial |
E.coli |
CSB-EP707843MO1 |
| PRKAG2 |
Q9UGJ0 |
Recombinant Human 5'-AMP-activated protein kinase subunit gamma-2(PRKAG2) |
E.coli |
CSB-EP887021HU |
| Prkab1 |
Q9R078 |
Recombinant Mouse 5-AMP-activated protein kinase subunit beta-1(Prkab1) |
E.coli |
CSB-EP889918MO |
Incretin and GLP-1 Receptor (Glucagon-like peptide-1 receptor): Related to insulin secretion and blood glucose regulation.
| Target |
Uniprot ID |
Product Name |
Source |
Code |
| Prkaa1 |
Q5EG47 |
Recombinant Mouse 5'-AMP-activated protein kinase catalytic subunit alpha-1(Prkaa1) |
Baculovirus |
CSB-BP707843MO |
| Prkaa1 |
Q5EG47 |
Recombinant Mouse 5-AMP-activated protein kinase catalytic subunit alpha-1(Prkaa1) |
Baculovirus |
CSB-BP707843MOa0 |
| PRKAB2 |
O43741 |
Recombinant Human 5'-AMP-activated protein kinase subunit beta-2(PRKAB2) |
E.coli |
CSB-EP527326HU |
| Prkag1 |
O54950 |
Recombinant Mouse 5-AMP-activated protein kinase subunit gamma-1(Prkag1) |
E.coli |
CSB-EP528783MO |
| Prkaa1 |
Q5EG47 |
Recombinant Mouse 5-AMP-activated protein kinase catalytic subunit alpha-1(Prkaa1),partial |
E.coli |
CSB-EP707843MO1 |
| PRKAG2 |
Q9UGJ0 |
Recombinant Human 5'-AMP-activated protein kinase subunit gamma-2(PRKAG2) |
E.coli |
CSB-EP887021HU |
| Prkab1 |
Q9R078 |
Recombinant Mouse 5-AMP-activated protein kinase subunit beta-1(Prkab1) |
E.coli |
CSB-EP889918MO |
Adiponectin: A hormone secreted by adipocytes, involved in glucose metabolism and insulin sensitivity.
| Target |
Uniprot ID |
Product Name |
Source |
Code |
| ADIPOQ |
Q3Y5Z3 |
Recombinant Bovine Adiponectin(ADIPOQ) |
E.coli |
CSB-EP661101BO |
| Adipoq |
Q60994 |
Recombinant Mouse Adiponectin(Adipoq) |
E.coli |
CSB-EP723362MO |
| Adipoq |
Q60994 |
Recombinant Mouse Adiponectin(Adipoq),partial |
E.coli |
CSB-RP079474m |
| ADIPOQ |
Q3Y5Z3 |
Recombinant Bovine Adiponectin(ADIPOQ) |
Yeast |
CSB-YP661101BO |
| ADIPOQ |
F7DZE7 |
Recombinant Horse Adiponectin D(ADIPOQ),partial |
E.coli |
CSB-EP4093HO |
NF-κB (Nuclear Factor κB): Associated with chronic inflammation and diabetes.
| Target |
Uniprot ID |
Product Name |
Source |
Code |
| NFKB1 |
P19838 |
Recombinant Human Nuclear factor NF-kappa-B p105 subunit(NFKB1) |
E.coli |
CSB-EP015759HU |
| NFKB1 |
P19838 |
Recombinant Human Nuclear factor NF-kappa-B p105 subunit(NFKB1),partial |
E.coli |
CSB-EP015759HU1 |
SREBP (Sterol Regulatory Element-Binding Protein): Associated with lipid metabolism and insulin resistance.
These proteins play crucial roles in the pathogenesis, diagnosis, treatment, and prevention of diabetes. Studying these proteins contributes to a better understanding of the biological basis of diabetes and the development of new treatment methods and interventions.
6. Recent Research and Advances
(Adu et al., 2019) studied enablers and barriers to effective diabetes self-management through an international online survey and telephone interviews. Common enablers included the will to prevent complications and the use of technological devices [13].
The American Diabetes Association (ADA) annually updates its Standards of Medical Care in Diabetes. The 2019 standards, developed by the committee (Chamberlain et al., 2019), provide evidence-based recommendations for the diagnosis and management of diabetes [14].
(Khan et al., 2019) classified diabetes into three types: type 1, type 2 diabetes mellitus (T2DM), and gestational diabetes. Their study covers the diagnosis, treatments, and translational research
[15].
(Vasu et al., 2019) reviewed studies tracking miRNAs in blood for type 1 diabetes, obesity, pre-diabetes, type 2 diabetes, and gestational diabetes. The findings suggest miRNA signatures can stage the progression of different diabetes modes [16].
(Zhang et al., 2021) discussed monogenic diabetes, accounting for 1%-5% of diabetes, covering various types such as maturity-onset diabetes of the young and syndromic diabetes [17].
(Lin et al., 2021) aimed to review the evidence, user experience, and cost-effectiveness of continuous glucose monitoring (CGM), emphasizing improved satisfaction in people with diabetes [18].
References:
[1] Wild S, Roglic G, Green A, et al. Global Prevalence of Diabetes [J]. Diabetes Care, 2004, 27(5): 1047-1053.
[2] Devendra D, Liu E, Eisenbarth G S. Type 1 diabetes: recent developments [J]. Bmj British Medical Journal, 2004, 328(7442): 750-754.
[3] Gan M J, Albanese-O’Neill A, Haller M J. Type 1 Diabetes: Current Concepts in Epidemiology, Pathophysiology, Clinical Care, and Research [J]. Current Problems in Pediatric & Adolescent Health Care, 2012, 42(10): 269-291.
[4] Hummel K, Mcfann K K, Realsen J, et al. The Increasing Onset of Type 1 Diabetes in Children [J]. Journal of Pediatrics, 2012, 161(4): 652-657.
[5] Imkampe A K, Gulliford M C. Trends in Type 1 diabetes incidence in the UK in 0-to 14-year-olds and in 15-to 34-year-olds, 1991-2008 [J]. Diabetic Medicine, 2011, 28(7): 811-814.
[6] Harris S B, Caulfield L E, Sugamori M E, et al. The epidemiology of diabetes in pregnant Native Canadians. A risk profile [J]. Diabetes Care, 1997, 20(9): 1422-1425.
[7] Fridlyand L E, Philipson L H. Does the glucose-dependent insulin secretion mechanism itself cause oxidative stress in pancreatic beta-cells [J]. Diabetes, 2004, 53(8): 1942-8.
[8] Pociot F, Akolkar B, Concannon P, et al. Genetics of type 1 diabetes: what's next? [J]. Diabetes, 2010, 59(7): 1561.
[9] Borchers A T, Naguwa S M, Shoenfeld Y, et al. The geoepidemiology of systemic lupus erythematosus [J]. Autoimmunity Reviews, 2010, 9(5): A277-A287.
[10] Brewster J L, Valoir T D, Dwyer N D, et al. An Osmosensing Signal Transduction Pathway in Yeast [J]. Science, 1993, 259(5102): 1760-1763.
[11] Osman N, Ballinger M L, Dadlani H M, et al. p38 MAP kinase mediated proteoglycan synthesis as a target for the prevention of atherosclerosis [J]. Cardiovascular & Haematological Disorders-Drug Targets, 2008, 8(4): 287-292.
[12] Kato A, Okura T, Hamada C, et al. Cell Stress Induces Upregulation of Osteopontin via the ERK Pathway in Type II Alveolar Epithelial Cells [J]. Plos One, 2014, 9(6): e100106.
[13] Mary D Adu, Usman H Malabu, Aduli E O Malau-Aduli, et al. "Enablers And Barriers To Effective Diabetes Self-management: A Multi-national Investigation", PLOS ONE, 2019.
[14] James J Chamberlain, Kacie Doyle-Delgado, Lacie Peterson, Neil Skolnik, "Diabetes Technology: Review Of The 2019 American Diabetes Association Standards Of Medical Care In Diabetes", ANNALS OF INTERNAL MEDICINE, 2019.
[15] Radia Marium Modhumi Khan, Zoey Jia Yu Chua, Jia Chi Tan, et al. "From Pre-Diabetes to Diabetes: Diagnosis, Treatments and Translational Research", MEDICINA (KAUNAS, LITHUANIA), 2019.
[16] Srividya Vasu, Kenjiro Kumano, Carly M Darden, et al. "MicroRNA Signatures As Future Biomarkers For Diagnosis Of Diabetes States", CELLS, 2019.
[17] Haichen Zhang, Kevin Colclough, Anna L Gloyn, Toni I Pollin, "Monogenic Diabetes: A Gateway to Precision Medicine in Diabetes", THE JOURNAL OF CLINICAL INVESTIGATION, 2021.
[18] Rose Lin, Fran Brown, Steven James , et al. "Continuous Glucose Monitoring: A Review of The Evidence in Type 1 and 2 Diabetes Mellitus", DIABETIC MEDICINE : A JOURNAL OF THE BRITISH DIABETIC , 2021.
CUSABIO team. Diabetes: What You Need to Know Before Starting Research?. https://www.cusabio.com/c-19000.html





Comments
Leave a Comment