[1] Chatterjee, Subhajit, Saptarshi Sinha, and Chanakya Nath Kundu. "Nectin cell adhesion molecule-4 (NECTIN-4): a potential target for cancer therapy." European Journal of Pharmacology 911 (2021): 174516.
[2] Hashimoto, Hiroki, et al. "Nectin-4: A novel therapeutic target for skin cancers." Current Treatment Options in Oncology 23.4 (2022): 578-593.
[3] Cabaud, Olivier, et al. "Overcoming Resistance to Anti–Nectin-4 Antibody-Drug Conjugate." Molecular Cancer Therapeutics 21.7 (2022): 1227-1235.
[4] Challita-Eid, Pia M., et al. "Enfortumab vedotin antibody–drug conjugate targeting nectin-4 is a highly potent therapeutic agent in multiple preclinical cancer models." Cancer research 76.10 (2016): 3003-3013.
[5] Li, Kaiyue, et al. "Therapeutic prospects of nectin-4 in cancer: applications and value." Frontiers in Oncology 14 (2024): 1354543.
[6] Kedashiro, Shin, et al. "Nectin-4 and p95-ErbB2 cooperatively regulate Hippo signaling-dependent SOX2 gene expression, enhancing anchorage-independent T47D cell proliferation." Scientific Reports 11.1 (2021): 7344.
[7] Sethy, Chinmayee, et al. "Nectin-4 promotes lymphangiogenesis and lymphatic metastasis in breast cancer by regulating CXCR4-LYVE-1 axis." Vascular Pharmacology 140 (2021): 106865.
[8] Chatterjee, Subhajit, Saptarshi Sinha, and Chanakya Nath Kundu. "Nectin cell adhesion molecule-4 (NECTIN-4): a potential target for cancer therapy." European Journal of Pharmacology 911 (2021): 174516.
[9] Liu, Yongheng, et al. "Role of Nectin‑4 protein in cancer." International Journal of Oncology 59.5 (2021): 1-14.
[10] Zhang, Jinxiu, et al. "Upregulation of nectin‑4 is associated with ITGB1 and vasculogenic mimicry and may serve as a predictor of poor prognosis in colorectal cancer." Oncology Letters 18.2 (2019): 1163-1170.
[11] Deng, Haifeng, et al. "Over-expression of Nectin-4 promotes progression of esophageal cancer and correlates with poor prognosis of the patients." Cancer Cell International 19 (2019): 1-13.
[12] Zhang, Yan, et al. "High expression of Nectin-4 is associated with unfavorable prognosis in gastric cancer." Oncology letters 15.6 (2018): 8789-8795.
[13] Zhang, Yijian, et al. "A novel PI3K/AKT signaling axis mediates Nectin-4-induced gallbladder cancer cell proliferation, metastasis and tumor growth." Cancer letters 375.1 (2016): 179-189.
[14] Chatterjee, Subhajit, and Chanakya Nath Kundu. "Nanoformulated quinacrine regulates NECTIN-4 domain specific functions in cervical cancer stem cells." European Journal of Pharmacology 883 (2020): 173308.
[15] DeRycke, Melissa S., et al. "Nectin 4 overexpression in ovarian cancer tissues and serum: potential role as a serum biomarker." American journal of clinical pathology 134.5 (2010): 835-845.
[16] Takano, Atsushi, et al. "Identification of nectin-4 oncoprotein as a diagnostic and therapeutic target for lung cancer." Cancer research 69.16 (2009): 6694-6703.
[17] Heath, Elisabeth I., and Jonathan E. Rosenberg. "The biology and rationale of targeting nectin-4 in urothelial carcinoma." Nature Reviews Urology 18.2 (2021): 93-103.
[18] Fabre-Lafay, Stéphanie, et al. "Nectin-4 is a new histological and serological tumor associated marker for breast cancer." BMC cancer 7 (2007): 1-16.
[19] Ma, Jie, et al. "Expression and clinical significance of Nectin-4 in hepatocellular carcinoma." OncoTargets and therapy (2016): 183-190.
[20] Sanders, Christine, et al. "Nectin-4 is widely expressed in head and neck squamous cell carcinoma." Oncotarget 13 (2022): 1166.
[21] Toda, Soji, et al. "TROP-2, Nectin-4, GPNMB, and B7-H3 are potentially therapeutic targets for anaplastic thyroid carcinoma." Cancers 14.3 (2022): 579.
[22] Tanaka, Yuka, et al. "NECTIN4: A novel therapeutic target for melanoma." International journal of molecular sciences 22.2 (2021): 976.
[23] Tanaka, Yuka, et al. "Nectin cell adhesion molecule 4 (NECTIN4) expression in cutaneous squamous cell carcinoma: a new therapeutic target?." Biomedicines 9.4 (2021): 355.

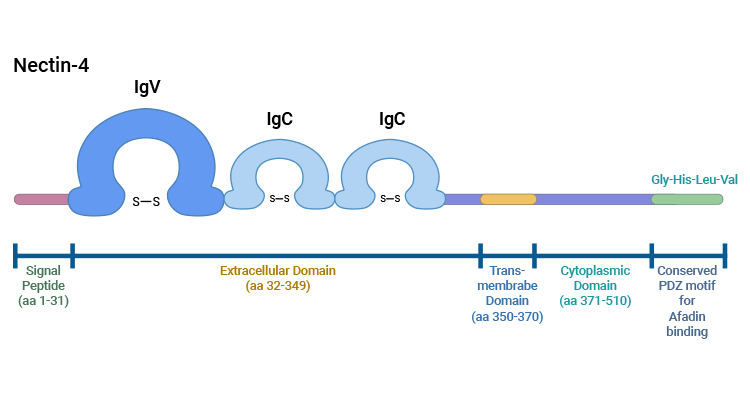
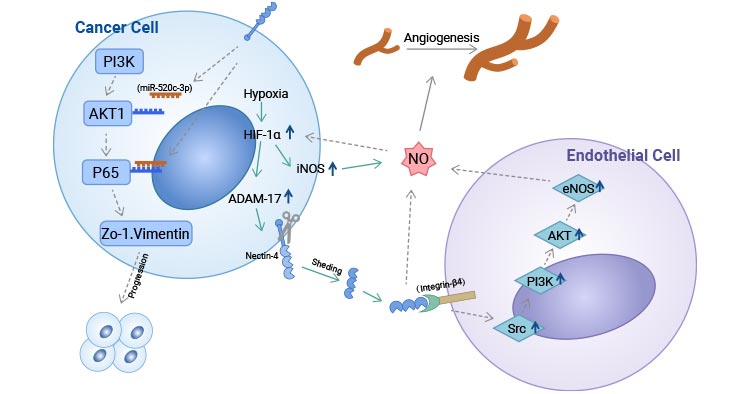
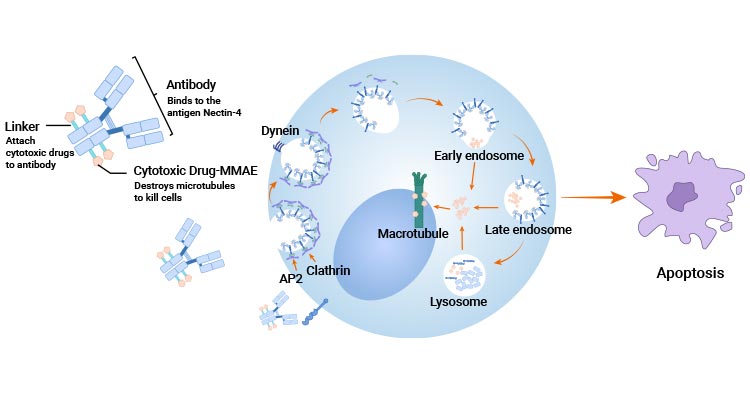



Comments
Leave a Comment