1. What is MICB
1.1 Molecular structure and localization
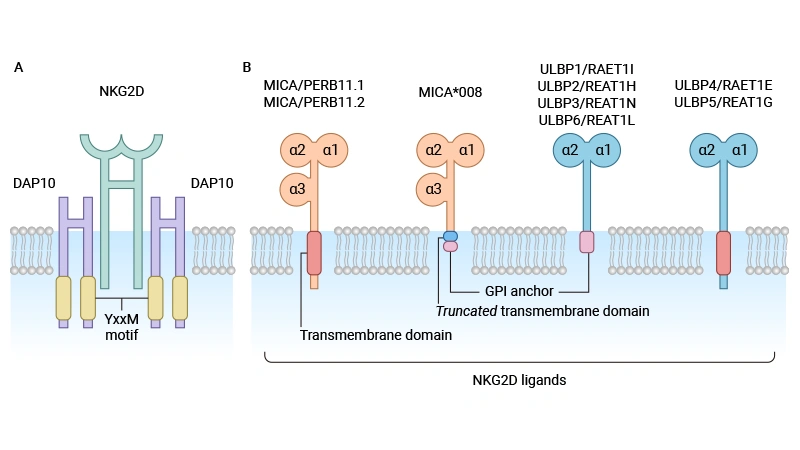
Major histocompatibility complex class I chain-related protein B (MICB) is located on the short arm of human chromosome 6 (6p21.33) and is a core member of the MHC class I chain-associated molecule (MIC) family. This gene cluster is adjacent to the HLA-B locus and contains two major functional genes, MICA and MICB, which share 84% homology at the protein level. Unlike classical MHC class I molecules, MICB does not bind to β2 microglobulin and does not have an antigenic peptide presentation function, but is expressed in a state of cellular stress as a ligand-specific activation of NKG2D, a key receptor for the innate immune system, thereby initiating immune surveillance mechanisms [1].
The MICB protein contains three highly conserved domains: extracellular α1 and α2 domains constitute the binding interface with NKG2D; The α3 domain folds like an immunoglobulin to ensure molecular stability; The transmembrane zone anchors it to the cell membrane. In addition, MICB has a long 3'-untranslated region and highly polymorphic genes, with more than 225 alleles identified, such as the new alleles MICB005:06 and MICB026 in the Han population in southern China [1,2].
Figure: Structure of NKG2D receptor and homologous ligand [1]
1.2 Biological function
Under normal physiological conditions, MICB is hardly expressed on the surface of healthy cells; However, its expression is significantly upregulated when cells are exposed to pathological stimuli such as DNA damage, viral infection, heat shock, or oxidative stress [3]. As a ligand for NKG2D, MICB is predominantly expressed on the surface of stressed cells, and by binding to NKG2D on the surface of NK cells, CD8+ T cells, γδ T cells, and activated macrophages, it activates innate and adaptive immune responses, prompting effector cells to exert cytotoxicity and secrete cytokines (e.g., IFN-γ). This mechanism is particularly critical in tumor immune surveillance, where MICB-positive tumor cells can be recognized and eliminated by the immune system, forming the body's first line of defense against tumors [1,2,4].
2. Regulatory mechanism and signaling pathway of MICB
2.1 Expression regulation mechanisms
● Transcription and Epiregulation
Single nucleotide polymorphisms (SNPs) in the promoter region of MICB can affect its transcriptional activity. For example, the rs1051788 (G406A) variant results in the substitution of the amino acid D136N of the MICB protein, reducing cell surface MICB expression and NKG2D binding capacity [3]. In addition, microRNA-34a (miR-34a) can dually promote MICB expression by inhibiting the transcription factor E2F1 and activating ATR kinase, but its effect is dependent on intracellular basal levels of E2F1 [5].
● Post-translational modification and clipping
In the tumor microenvironment, the expression of MICB is dynamically regulated by a variety of factors. At the gene level, DNA damage activates the ATM/ATR kinase pathway, which directly upregulates MICB transcription; At the epigenetic level, the methylation status of the promoter region significantly affected its expression level. In addition, transcription factors such as heat shock factor 1 (HSF1) and nuclear factor κB (NF-κB) directly bind to the MICB promoter and promote its expression.
In addition to transcriptional regulation, tumor cells can evade immune surveillance through post-translational modifications. For example, the metalloproteinases ADAM10 and ADAM17 are used to hydrolyze the MICB transmembrane region, releasing soluble MICB (sMICB) and reducing cell surface ligand density [4,6]. At the same time, sMICB continuously binds to NKG2D on the NK cell surface, inducing receptor endocytosis and degradation, resulting in inhibition of NK cell function. Clinical data have shown that serum sMICB levels are significantly elevated in patients with multiple cancers, such as melanoma and prostate cancer, and are closely related to disease progression and poor prognosis. In addition, tumor cells can directly inhibit MICB promoter activity and reduce MICB expression through epigenetic silencing, the autophagy-lysosomal pathway, or by utilizing oncogenes (e.g., RAS, MYC) [6].
2.2 NKG2D-MICB signaling pathway
● Activation Pathway:
When MICB binds to NKG2D, it triggers a specific signaling cascade within different immune cells. In NK cells, the NKG2D intracellular region signals activation via the adaptor protein DAP10. DAP10 contains a YINM motif that recruits and phosphorylates the PI3K and Grb2-Vav1 pathways upon activation, ultimately upregulates NF-κB and MAPK signaling, and promotes the secretion of cytokines (e.g., IFN-γ, TNF-α) and the release of cytotoxic granules (perforin, granzyme). In CD8+ T cells, the MICB-NKG2D signal does not directly activate the T cell receptor (TCR), but acts as a costimulatory signal to enhance TCR-mediated cell activation and proliferation, and inhibit activation-induced cell death (AICD), effectively prolonging the survival time of effector T cells.
● Inhibition pathway:
As a key molecule in tumor immune evasion, sMICB binds to NKG2D to induce receptor endocytosis and degradation and reduce effector cell activity. For example, serum levels of sMICB in dengue patients are positively correlated with disease severity, suggesting that it plays an important role in immunosuppression [7].
3. MICB-related diseases
3.1 Neoplastic diseases
Aberrant expression of MICB affects tumor progression, metastasis and drug resistance. In metastatic melanoma and triple-negative breast cancer, high expression of ADAM17 in tumor cells promotes MICB shedding, leading to micrometastatic escape. A 2022 Nature study showed that mice vaccinated with MICB after surgery had a 70%-80% reduction in lung metastases, which was associated with a 29.3-fold, 17.9-fold, and 38.9-fold increase in CD4+ T, CD8+ T, and NK cell infiltration, respectively [8].
In drug-resistant tumor models such as MHC-I, MHC-II, or IFN-γ receptor deficiencies, targeted MICB vaccines activate CD4+ T and NK cells, enabling long-term tumor-free survival in 50%-75% of mice. Mechanistically, CD4+ T cells secrete IFN-γ to activate dendritic cells and recruit NK cells to kill tumors. In addition, high MICB expression in colorectal cancer predicts a good prognosis; In multiple myeloma, high expression is associated with poor prognosis due to increased sMICB [6,9]. Liver cancer and gastric cancer evade immune surveillance through E2F1 transcription inhibition and STAT3 downregulation [5,10].
3.2 Inflammation and immune-related diseases
● Autoimmune diseases: MICB gene variants are significantly associated with systemic sclerosis (SSc). High expression of MICB in skin cells of SSc patients activates NKG2D signaling and promotes T cell infiltration and collagen deposition. Mutations in the interferon pathway gene and synergistic presence in the NOTCH4 gene exacerbate the risk of morbidity.
● Acute lung injury (ALI) and primary graft dysfunction (PGD): lung transplant donors carrying the MICB rs1051788 AA genotype reduce the risk of severe PGD by 11.1% [3].
● Infectious diseases: in dengue patients, the MICB rs3132468 variant is associated with a risk of severe disease or inhibition of NKG2D by sMICB [7].
3.3 Chronic kidney disease
In end-stage renal disease, tubular cell inflammation induces high expression of MICB, and the released sMICB binds to NK cell NKG2D to degrade it, weakening immune function and exacerbating renal function deterioration. Serum sMICB levels in hemodialysis patients were positively correlated with the inflammatory marker CRP, and decreasing sMICB improved the microinflammatory state.
3.4 Hematopoietic Stem Cell Transplantation (HSCT)
In HSCT, donor-recipient MICB mismatches are associated with an increased risk of acute graft-versus-host disease (aGVHD), particularly in the context of MICB*005:02 allele mismatches, where NK cell-mediated immune responses may exacerbate tissue damage.
4. Progress in drug research and development based on MICB
At present, there are no drugs based on MICB targets, and a number of drugs are in the preclinical or clinical research stage, with the highest clinical phase 1 clinical trial, and positive clinical results have been announced, and some of the projects under development are listed as follows:
| Drugs |
Mechanism of action |
Type of medication |
Indications under investigation (disease name) |
Institutions under research |
Highest R&D stage |
| DM-919 |
MICA inhibitor | MICB inhibitors |
Monoclonal antibodies |
Advanced malignant solid tumors |
Danma (Suzhou) Biomedical Technology Co., Ltd |
Phase 1 clinical trial |
| CLN-619 |
MICA inhibitor | MICB inhibitors |
Monoclonal antibodies |
Recurrent multiple myeloma | Advanced malignant solid tumors | Non-small cell lung cancer | pancreatic cancer |
Cullinan Oncology LLC |
Phase 1 clinical trial |
| AHA-1031 |
MICA inhibitor | MICB inhibitors |
Bispecific antibodies |
STK11-mutated non-small cell lung cancer |
The University of Texas Southwestern Medical Center | Aakha Biologics | Alloy Therapeutics, Inc. |
Preclinical |
| FT-836 |
MICA inhibitor | MICB inhibitors |
CAR-T |
Solid tumors | tumor |
Fate Therapeutics, Inc. | Dana-Farber Cancer Institute, Inc. |
Preclinical |
| MICA/BxCD3(Xencor Inc.) |
CD3 stimulator | MICA inhibitor | MICB inhibitors |
Bispecific T cell binder |
tumor |
Xencor, Inc. |
Preclinical |
| SYB-010 |
MICA inhibitor | MICB inhibitors |
Monoclonal antibodies |
tumor |
CanCure LLC |
Preclinical |
| Tri-modal CAR+TCR+hnCD16+iPSC-derived T cells(Fate) |
BCMA inhibitor | CD16a modulator | MICA inhibitor | MICB inhibitor | NY-ESO-1 inhibitors |
Induced pluripotent stem cells | CAR-T |
Solid tumors |
Fate Therapeutics, Inc. |
Preclinical |
| BSI-120 |
MICA inhibitor | MICB inhibitors |
Monoclonal antibodies |
tumor |
Biosion Biotechnology (Nanjing) Co., Ltd |
Preclinical |
| B10G5 |
MICA inhibitor | MICB inhibitor | Natural killer cell modulator |
Monoclonal antibodies |
Multiple myeloma | Metastatic prostate cancer |
Severance Hospital | CanCure LLC |
Preclinical |
| ADI-925 |
MICA inhibitor | MICB inhibitor | ULBP1 inhibitors |
General purpose CAR-T |
Solid tumors |
Adicet Therapeutics, Inc. |
Preclinical |
| GenSci-P107 |
MICA inhibitor | MICB inhibitors |
Bispecific antibodies |
Hepatocellular carcinoma | Non-small cell lung cancer | Colorectal cancer | gastric cancer |
Changchun Jinsai Pharmaceutical Co., Ltd |
Preclinical |
5. MICB related products
As a key ligand for NKG2D, the balance of expression and shear of MICB regulates immune surveillance and escape. Current studies have revealed its role in tumors, ALI, and infectious diseases, and antibody drugs targeting MICB cleavage have demonstrated clinical potential.
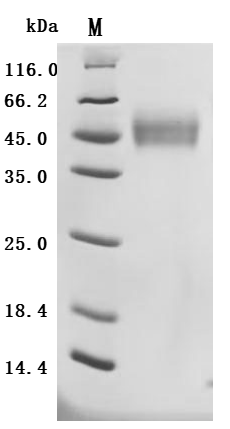
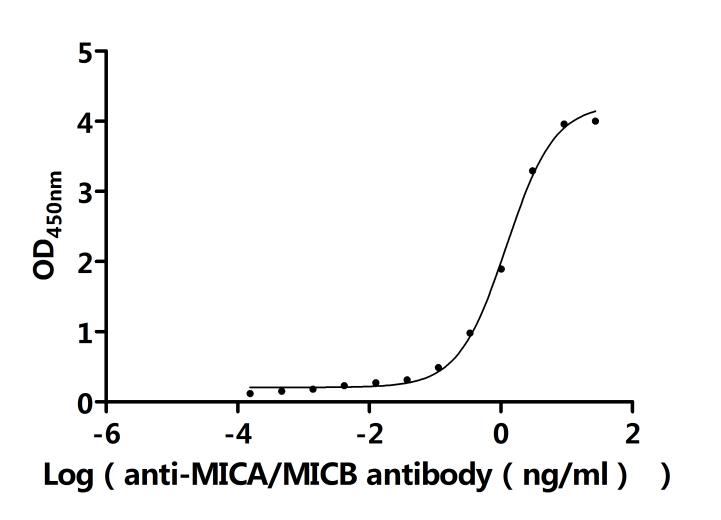
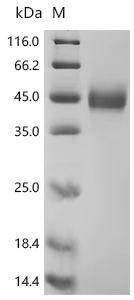
CUSABIO provides recombinant proteins and ELISA kits related to MICB research to assist you in conducting MICB mechanism or clinical research.
► Click here to view all products related to MICB
References
[1] Fang, Z.W., et al. (2021). A novel MICB allele, MICB*004:02, identified in a western china Uyghur individual. HLA.
[2] Liu, X., et al. (2012). MICB polymorphism in a southern Chinese Han population. Human Immunology.
[3] Aguilar, O.A., et al. (2024). MICB Genomic Variant Is Associated with NKG2D-mediated Acute Lung Injury and Death. Am J Respir Crit Care Med.
[4] Ferrari de Andrade, L., et al. (2018). Antibody-mediated inhibition of MICA and MICB shedding promotes NK cell-driven tumor immunity. Science.
[5] Zhou, M.T., et al. (2018). MicroRNA-34a Promotes MICB Expression in Hepatocytes. Carcinogenesis.
[6] Jinushi, M., et al. (2008). MHC class I chain-related protein A antibodies and shedding are associated with the progression of multiple myeloma. Proc Natl Acad Sci U S A.
[7] Khor, C.C., et al. (2011). Genome-wide association study identifies susceptibility loci for dengue shock syndrome at MICB and PLCE1. Nat Genet.
[8] Soumya Badrinath, et al. (2022). A vaccine targeting resistant tumours by dual T cell plus NK cell attack. Nature.
[9] Yu, S. (2019). High MICB expression confers prognostic benefit in colorectal cancer. Annals of Oncology.
[10] Li, Y., et al. (2010). MICB0106 gene polymorphism is associated with ulcerative colitis in central China. Int J Colorectal Dis.
[11] Eric Alves, et al. (2021). Manipulating the NKG2D Receptor-Ligand Axis Using CRISPR: Novel Technologies for Improved Host Immunity. Front Immunol.
CUSABIO team. MICB: a key molecule that bridges cellular stress and immune responses. https://www.cusabio.com/c-21242.html



Comments
Leave a Comment