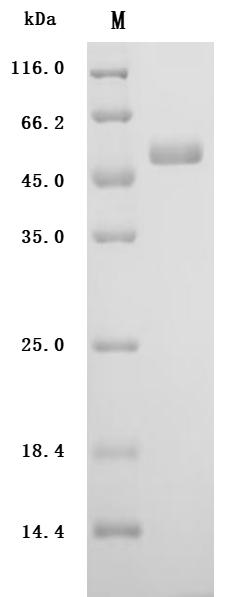
The recombinant human Urokinase-type plasminogen activator (PLAU) production involves several key steps to ensure high purity, low endotoxin levels, and verified biological activity. First, the PLAU gene fragment (21-431aa) is cloned into a mammalian expression vector with a C-terminal 10xHis tag gene. The construct is transfected into suitable mammalian cells for expression optimization. After protein expression, the PLAU protein is purified using Ni-NTA affinity chromatography to achieve >95% purity, as confirmed by SDS-PAGE. Endotoxin levels are removed using endotoxin removal resins and reduced to <1.0 EU/μg measured by the LAL method. The harvested recombinant PLAU protein is tested for enzymatic activity by cleaving the substrate Z-GGR-AMC, with a specific activity >2000 pmol/min/μg.
Human PLAU (uPA) is a serine protease in the plasminogen activation system, which is essential for various physiological and pathological processes, including fibrinolysis, tissue remodeling, and cell migration.
The activation of uPA occurs when it binds to its specific receptor, the urokinase-type plasminogen activator receptor (uPAR), which is anchored to the cell membrane via a glycosyl-phosphatidylinositol (GPI) tail [4][5]. Activated uPA catalyzes the conversion of inactive plasminogen into plasmin, an active serine protease that degrades fibrin and other extracellular matrix components [1][2]. This enzymatic activity is important for processes such as wound healing, angiogenesis, and tumor invasion, where the breakdown of the extracellular matrix is necessary for cell movement and tissue restructuring [2][3]. This interaction also initiates intracellular signaling pathways that influence cell adhesion, migration, and proliferation [5][6].
The uPA-uPAR system is particularly significant in cancer biology, as it is often overexpressed in various tumors, contributing to cancer cell invasiveness and metastasis [3][6]. The regulation of uPA activity is tightly controlled by plasminogen activator inhibitors (PAIs), particularly PAI-1 and PAI-2, which inhibit uPA and thus modulate its proteolytic activity [7]. This regulation is vital in maintaining a balance between tissue remodeling and pathological conditions such as cancer progression and chronic inflammation [3][7].
References:
[1] E. Atkins, S. Zamora, B. Candia, A. Baca, & R. Orlando. Development of a mammalian suspension culture for expression of active recombinant human urokinase-type plasminogen activator, Cytotechnology, vol. 49, no. 1, p. 25-37, 2005. https://doi.org/10.1007/s10616-005-4637-7
[2] H. Petersen, M. Hansen, S. Schousboe, & P. Andreasen. Localization of epitopes for monoclonal antibodies to urokinase‐type plasminogen activator, European Journal of Biochemistry, vol. 268, no. 16, p. 4430-4439, 2001. https://doi.org/10.1046/j.1432-1327.2001.02365.x
[3] W. Zhang, D. Liu, J. Tan, J. Zhang, & L. Li. Expression of urokinase plasminogen activator and plasminogen activator inhibitor type-1 in ovarian cancer and its clinical significance, Oncology Reports, vol. 29, no. 2, p. 637-645, 2012. https://doi.org/10.3892/or.2012.2148
[4] F. Blasi and N. Sidénius. The urokinase receptor: focused cell surface proteolysis, cell adhesion and signaling, Febs Letters, vol. 584, no. 9, p. 1923-1930, 2009. https://doi.org/10.1016/j.febslet.2009.12.039
[5] L. Qin, L. Wang, J. Zhang, H. Zhou, Z. Yang, Y. Wang, et al. Therapeutic strategies targeting upar potentiate anti–pd-1 efficacy in diffuse-type gastric cancer, Science Advances, vol. 8, no. 21, 2022. https://doi.org/10.1126/sciadv.abn3774
[6] S. Nozaki, Y. Endo, H. Nakahara, K. Yoshizawa, T. Ohara, & E. Yamamoto. Targeting urokinase-type plasminogen activator and its receptor for cancer therapy, Anti-Cancer Drugs, vol. 17, no. 10, p. 1109-1117, 2006. https://doi.org/10.1097/01.cad.0000231483.09439.3a
[7] M. Wyganowska‐Świątkowska, A. Surdacka, E. Skrzypczak‐Jankun, & J. Jankun. The plasminogen activation system in periodontal tissue (review), International Journal of Molecular Medicine, vol. 33, no. 4, p. 763-768, 2014. https://doi.org/10.3892/ijmm.2014.1653