[1] Pflugh, David L., Stephen E. Maher, and Alfred LM Bothwell. "Ly-6I, a new member of the murine Ly-6 superfamily with a distinct pattern of expression. "The Journal of Immunology 165.1 (2000): 313-321.
[2] Guo, Quanyang, et al. "Identification and expression of an uncharacterized Ly-6 gene cluster in zebrafish Danio rerio." Functional & integrative genomics 15 (2015): 577-585.
[3] Rathbun, Luke, Anthony Magliocco, and Anil K. Bamezai. "Upregulated Ly-6 gene expression is associated with poor overall survival in uterine corpus endometrial carcinoma patients." The Journal of Immunology 208.1_Supplement (2022): 179-05.
[4] Swamynathan, Sudha, et al. "Secreted Ly-6/uPAR-related protein-1 (SLURP1) is a pro-differentiation factor that stalls G1-S transition during corneal epithelial cell cycle progression." The Ocular Surface 24 (2022): 1-11.
[5] Bamezai, Anil K., and Julie M. Miwa. "Biology of Ly-6 Supergene Family in Health and Disease. "Frontiers in Cell and Developmental Biology 10 (2022).
[6] Upadhyay, Geeta. "Emerging role of lymphocyte antigen-6 family of genes in cancer and immune cells. "Frontiers in immunology 10 (2019): 819.
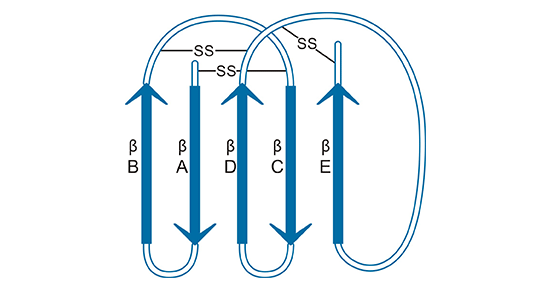
[7] Beliakov, I. S., T. A. Karakasheva, and N. N. Mazurenko. "Exon-intron structure of the LY6G6D gene. "Molecular biology 43 (2009): 543-551.
[8] Saito, Suguru, et al. "Lymphocyte antigen 6 complex locus G6D downregulation is a novel parameter for functional impairment of neutrophils in aged mice. " Frontiers in Immunology 13 (2022).
[9] Lee, Pui Y., et al. "Ly6 family proteins in neutrophil biology." journal of leukocyte biology 94.4 (2013): 585-594.
[10] Tan, Peng, et al. "Myeloid loss of Beclin 1 promotes PD-L1 hi precursor B cell lymphoma development." The Journal of clinical investigation 129.12 (2022) : 5261-5277. : 5261-5277.
[11] Caruso, Francesca Pia, et al. "Lymphocyte antigen 6G6D-mediated modulation through p38α MAPK and DNA methylation in colorectal cancer." Cancer Cell International 22.1 (2022): 1-13.
[12] Willuda, Joerg, et al. "Preclinical anti-tumor efficacy of an anti-C4. 4a (LYPD3) antibody drug conjugate for the treatment of lung squamous cell carcinoma." Cancer Research 74.19_Supplement (2014): 5445-5445.
[13] Özhan, Günes, et al. "Lypd6 enhances Wnt/β-catenin signaling by promoting Lrp6 phosphorylation in raft plasma membrane domains." developmental cell 26.4 (2013): 331-345.
[14] Miwa, Julie M. "Lynx1 prototoxins: critical accessory proteins of neuronal nicotinic acetylcholine receptors." current opinion in pharmacology 56 ( 2021): 46-51.
[15] Broquet, Alexis, et al. "Depletion of natural killer cells increases mice susceptibility in a Pseudomonas aeruginosa pneumonia model." critical care medicine 42.6 (2014): e441-e450.
[16] Wang, Wenxiu, et al. "Exogenous interleukin-33 promotes hepatocellular carcinoma growth by remodelling the tumour microenvironment." journal of translational medicine 18 (2020): 1-15.
[17] Pfaender, Stephanie, et al. "LY6E impairs coronavirus fusion and confers immune control of viral disease." Nature microbiology 5.11 (2020): 1330-1339.
[18] Farkas, Imre, et al. "CD59 blocks not only the insertion of C9 into MAC but inhibits ion channel formation by homologous C5b-8 as well as C5b -9." The Journal of physiology 539.2 (2002): 537-545.
[19] McLaughlin, Barbara J., et al. "Novel role for a complement regulatory protein (CD46) in retinal pigment epithelial adhesion. "Investigative ophthalmology & visual science 44.8 (2003): 3669-3674.
[20] Giordano, Guido, et al. "JAK/Stat5-mediated subtype-specific lymphocyte antigen 6 complex, locus G6D (LY6G6D) expression drives mismatch repair proficient colorectal cancer." Journal of Experimental & Clinical Cancer Research 38.1 (2019): 1-11.
[21] Caruso, Francesca Pia, et al. "LY6G6D is Epigenetically Activated in Classical Colorectal Adenocarcinoma and Hyper-Methylated in Mucinous Subtypes Determining Resistance to FOLFOX Therapeutic Regimens."(2021).
[22] Batista-Duharte, Alexander, et al. "Immune Checkpoint Inhibitors for Vaccine Improvements: Current Status and New Approaches." Pharmaceutics 14.8 ( 2022): 1721.
[23] Zych, Michał, et al. "Differences in Immune Checkpoints Expression (TIM-3 and PD-1) on T Cells in Women with Recurrent Miscarriages- Preliminary Studies." Journal of Clinical Medicine 10.18 (2021): 4182.
[24] Liu, Jun, et al. "Immune-checkpoint proteins VISTA and PD-1 nonredundantly regulate murine T-cell responses. "Proceedings of the National Academy of Sciences 112.21 (2015): 6682-6687.

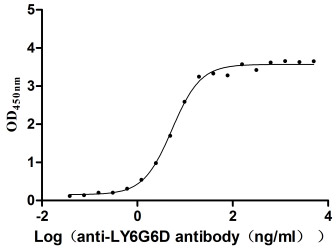
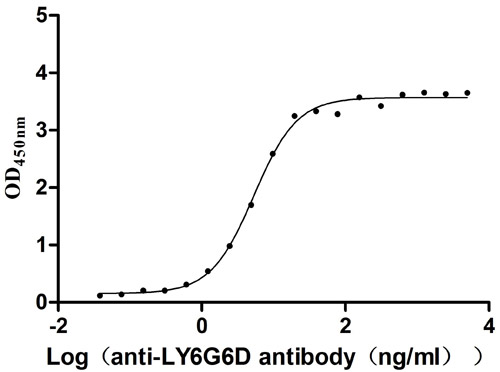
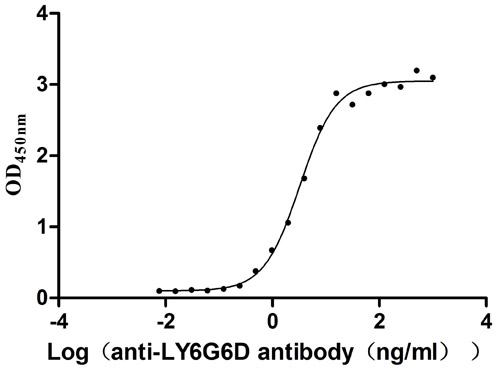
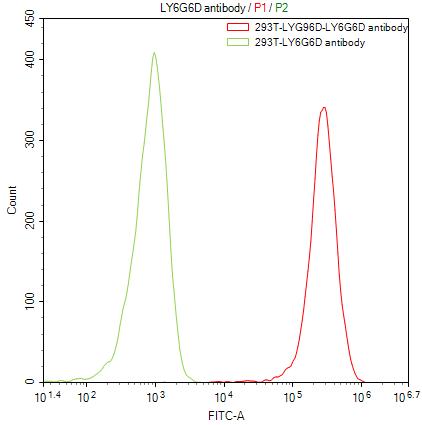



Comments
Leave a Comment