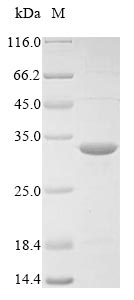
This recombinant Staphylococcus aureus sensor protein kinase walK gets expressed in E. coli and includes a partial protein length that corresponds to residues 382-600. The construct comes with an N-terminal 10xHis-tag and a C-terminal Myc-tag, which makes purification and detection more straightforward. SDS-PAGE analysis confirms the protein shows a purity level greater than 85%. This product is intended for research use only, with no reported endotoxin levels.
WalK appears to be a sensor protein kinase from Staphylococcus aureus that plays what seems like a crucial role in bacterial signal transduction. It's part of the two-component regulatory system WalKR, which is likely important for maintaining cell wall metabolism and homeostasis. Understanding how WalK works and gets regulated may provide insights into bacterial growth and survival mechanisms - something that's become a significant focus in microbiological and pharmaceutical research.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Staphylococcus aureus WalK is a bacterial histidine kinase that requires precise folding, proper ATP-binding domain formation, and specific dimerization for its autophosphorylation activity in two-component signaling. The E. coli expression system is homologous to this bacterial kinase, which increases the probability of correct folding. The kinase domain fragment (382-600aa) contains the catalytic core but lacks the N-terminal transmembrane and sensory domains. The dual N-terminal 10xHis-tag and C-terminal Myc-tag may sterically interfere with the protein's active site or dimerization interfaces. While the homologous expression system favors proper folding, the probability of correct folding with functional kinase activity requires experimental validation.
1. In Vitro Kinase Activity Assay Development
This application requires functional validation. WalK kinase activity depends on precise ATP-binding site formation and dimerization capability. If correctly folded and active (verified through autophosphorylation assays), the protein may be suitable for kinetic studies. If misfolded/inactive (unverified), kinase assays will yield biologically meaningless results. The tags may sterically interfere with ATP binding or dimerization.
2. Antibody Development and Validation
This application is highly suitable as antibody development relies on antigenic sequence recognition rather than functional kinase activity. The kinase domain fragment provides specific epitopes for generating antibodies against WalK. The high purity (>85%) ensures minimal contamination-related issues during immunization protocols.
3. Protein-Protein Interaction Studies
This application carries a significant risk without proper folding validation. WalK interactions with response regulators and other signaling proteins require precise tertiary structure. If correctly folded (verified), the protein may identify physiological interaction partners. If misfolded/unverified, there is a high risk of non-specific binding or failure to replicate genuine signaling complex formation.
4. Structural and Biophysical Characterization
These studies are essential for determining folding status. Techniques should include size-exclusion chromatography to assess dimerization state, circular dichroism spectroscopy to evaluate secondary structure, and thermal shift assays to determine stability. However, the dual tags may interfere with crystallization for high-resolution structural studies.
Final Recommendation & Action Plan
The homologous E. coli expression system provides favorable conditions for this bacterial kinase, but the kinase domain fragment and dual-tag configuration necessitate experimental validation before functional applications. Begin with Application 4 (Structural Characterization) to assess folding quality through SEC (dimerization analysis), CD spectroscopy, and validate kinase activity through autophosphorylation assays. Applications 1 and 3 require rigorous functional validation before proceeding. Application 2 (antibody development) can proceed immediately. Consider tag removal for critical functional studies to minimize potential steric interference. Always include appropriate activity controls and validate key findings with full-length WalK when possible for reliable signaling pathway research.