Monoclonal antibodies (mAbs) have revolutionized medicine, diagnostics, and research since their discovery. The monoclonal antibody market has seen remarkable growth, estimated to reach over $250 billion globally by 2028, driven by advancements in biotechnology and an increasing demand for targeted therapies. Furthermore, mAbs account for a significant portion of new drug approvals, with a 2021 report noting that nearly 20% of all FDA-approved biologics were monoclonal antibodies.
MAbs are being utilized more frequently in areas like research and diagnosis, therapeutic applications for cancer and immunological diseases, as well as pharmacy, leading to a significant demand for their production.
This article provides a detailed exploration of monoclonal antibody production, exploring the steps for preparing monoclonal antibodies, cutting-edge techniques for monoclonal antibody production, as well as their unique advantages and existing limitations.
Table of Contents
1. What Are Monoclonal Antibodies?
Monoclonal antibodies (mAb) are monovalent antibodies produced by a single B-lymphocyte clone, with high specificity and homogeneity.
Monoclonal Antibody vs. Polyclonal Antibody
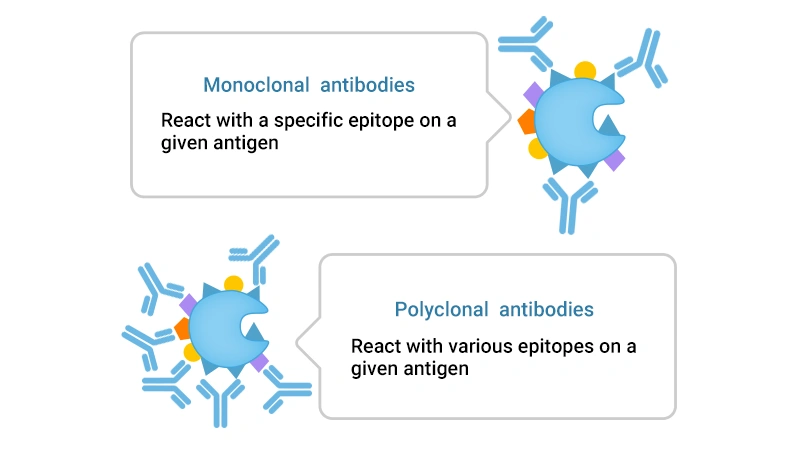
Figure 1. The main difference between monoclonal antibody and polyclonal antibody
Monoclonal antibodies are homogeneous and target the same epitope of an antibody, ensuring high specificity and reduced cross-reactivity. Monoclonal antibodies are consistent across batches, enabling reproducible results.
Polyclonal antibodies are heterogeneous and recognize multiple epitopes of an antigen.
Polyclonal antibodies show low specificity and are prone to cross-react with antigens with similar structures. There may be differences between each batch of antibodies prepared since polyclonal antibodies come from multiple B cell clones.
This may lead to false positive or false negative results.
Therefore, monoclonal antibodies are suitable for precise research and treatment needs (e.g. the development of therapeutic monoclonal antibody products).
2. How Is Monoclonal Antibody Produced?
Hybridoma technology is the most traditional and most commonly used method for monoclonal antibodies production. The monoclonal antibody production process through hybridoma technology usually includes the following main steps:
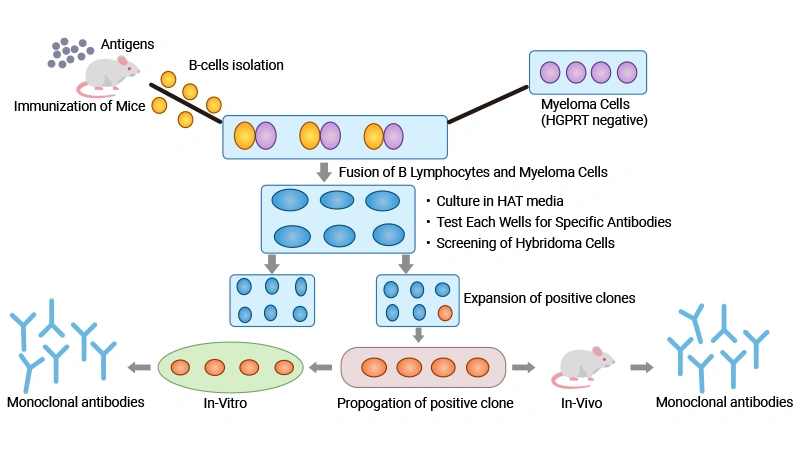
Figure 2. Diagram showing the production of monoclonal antibodies
This picture is cited from: https://pmc.ncbi.nlm.nih.gov/articles/PMC8521504/
2.1 Preparation of antigen-specific B cells
First, it is necessary to select a suitable antigen and immunize an animal (usually a mouse) with the antigen to induce B-lymphocytes to produce antigen-specific antibodies. It often takes multiple immunizations to enhance the immune response. Adjuvants may need to be added during the immunization process to improve the immune effect. The B-lymphocytes are then extracted from the spleen of the animal using density gradient centrifugation.
2.2 Cell fusion
The isolated B-lymphocytes are fused with immortal myeloma cells which lack the hypoxanthine-guanine-phosphoribosyl transferase (HGPRT) gene (encode the enzyme necessary for the salvage pathway of nucleic acids) and do not secrete any other immunoglobulin to form hybridoma cells by chemical method (use PEG) or electrofusion. Myeloma cells not containing the HGPRT gene are sensitive to HAT media, which is the preferred method in hybridoma technology [1].
2.3 Selection and cloning of specific hybridoma clones
These hybridoma cells are subsequently cultured in vitro in a medium containing hypoxanthine-aminopterin-thymidine (HAT) where only the B-lymphocyte-myeloma cell hybridomas can survive. This is due to their acquisition of immortality from the myeloma cells and selective HAT resistance from the primary B-lymphocytes [1].
The survived HAT-selection hybridomas are cloned using the "limiting dilution method" to screen out hybridoma cells that can produce desired antibodies. Each clone is derived from a single parent hybridoma cell containing a distinct primary B-lymphocyte and can therefore produce its own individual specific antibody. The most common screening techniques are ELISA and RIA.
2.4 Hybridoma clone amplification and antibody harvest
After the hybridoma clones with antigen specificity are selected, they are amplified by cell culture technology to produce a large number of monoclonal antibodies. Commercial hybridoma production of monoclonal antibodies generally uses the mouse ascites method. The antibodies harvested by the ascites method of monoclonal antibody production usually have high concentration, which makes the subsequent purification process more efficient.
Alternatively, hybridomas producing desired antibodies can be cultured in in vitro laboratory conditions. The in vitro method of monoclonal antibody production can obtain highly pure monoclonal antibodies [2,3].
2.5 Antibody purification
These collected antibodies can be isolated and purified from the mouse ascites or culture media by some purification techniques such as protein A affinity chromatography to obtain the final antibody products.
2.6 Antibody characterization and functional analysis
The purity and molecular weight of the antibody are analyzed by SDS-PAGE, WB, and other technologies. The bioactivity tests are used to evaluate the binding ability, inhibitory effect, and cytotoxicity of the antibody, ensuring that the monoclonal antibody meets the expected application requirements such as ELISA, WB, FC, IHC, etc.
2.7 Antibody storage
The produced monoclonal antibodies are cryopreserved to maintain their activity, usually using glycerol or DMSO as a protective agent.
Monoclonal vs Polyclonal Antibody Production
The process of generating monoclonal antibodies is complex and time-consuming (require 6 to 9 months). It is quite expensive. However, monoclonal antibody production can be performed on a large scale.
The polyclonal antibody production is relatively simple, usually including immunizing appropriate animals such as rabbits or goats, collecting animal serum, and extracting and purifying polyclonal antibodies from the anti-serum. The production of polyclonal antibodies is relatively fast (the final product can usually be obtained within a few weeks) and is low-cost.
3. Development of Monoclonal Antibody Production Technology
The history of monoclonal antibody technology can be traced back to the 1970s. In 1975, Köhler G. and Milstein C. successfully fused mouse B cells with myeloma cells for the first time, marking the birth of monoclonal antibody technology [4]. Since then, researchers have continuously improved and optimized this technology, which has greatly improved the production efficiency and specificity of monoclonal antibodies.
With the development of technology, the application scope of monoclonal antibodies has gradually expanded, from the initial laboratory research to clinical application, becoming an important tool for the treatment of various diseases such as cancer, infectious diseases, and autoimmune diseases.
Scientific progress and increasing demand drive advances in large-scale production of monoclonal antibodies.
| Technologies |
Description |
Advantages |
Disadvantages |
| Hybridoma technology |
Fusion of B cells with myeloma cells to produce antibody-producing hybridomas |
- Mature and widely used method
- High specificity and affinity
- Long-term, stable production
- Well-established, reproducible method
|
- Labor-intensive and time-consuming
- Requires immunization of animals
- Cell fusion rate is very low
- Mouse immunity may bring immunogenicity problems caused by species differences
|
| Recombinant antibody technology |
Cloning the antibody gene through genetic engineering techniques and then expressing it in the appropriate expression system such as mammalian cells, yeast, E.coli, etc. |
- Strong flexibility: can design different antibody structures, such as humanized antibody
- Rapid production: has a shorter production cycle and is the method for large scale monoclonal antibody production compared to the hybridoma technology
- High batch consistency: production process is standardized, with higher batch consistency, is suitable for industrial production
|
- High development cost
- Expression system limitations: different expression systems may affect the modification and folding of antibodies, resulting in affected activity and specificity
|
| Transgenic animal technology |
Introducing antibody genes into transgenic animals such as mice or goats to produce specific monoclonal antibodies in vivo |
- Efficient production: can continuously produce specific antibodies and is suitable for large-scale production
- Natural post-modification: Antibodies in transgenic animals usually undergo natural glycosylation and other modifications, which may have better activity and stability
- Can obtain stable antibody production strains through selective breeding
|
- High cost and time for animal development
- Ethical concerns about genetic modification
- Technically challenging
|
| Phage display technology |
Uses bacteriophages to display antibody fragments on their surface and then use screening methods to screen antibodies with specific binding ability from the cell bank |
- In vitro selection avoids reliance on animal immunity
- Allows high-throughput screening for high specificity and affinity
- Flexibility: applicable to the screening of various types of antibodies and antibody fragments
|
- Requires advanced expertise and equipment
- Antibodies may need additional steps for optimization and production
|
| Single B cell antibody technology |
Isolates and immortalizes antigen-specific B cells from humans or animals using microfluidics and single cell separation technology |
- High accuracy: can screen the best antibody-producing cells from a diverse B cell library and is highly targeted
- Rapid screening: can shorten the screening time and increase the success rate
|
- Technical complexity: requires sophisticated equipment and technology, difficult to operate
- Low cell survival rate: the cell survival rate may be low, affecting the final antibody yield
|
In short, transgenic animal technology is the in vivo method of monoclonal antibody production. Hybridoma technology and recombinant antibody technology are partially dependent on the in vivo animal process. While phage display technology and single B cell antibody technology are methods for in vitro monoclonal antibody production.
Conclusion
Monoclonal antibody production represents a blend of science, technology, and innovation that has profoundly impacted modern medicine and research. From hybridoma technology to cutting-edge recombinant methods, the journey of developing these powerful tools involves meticulous processes and continuous advancements. While challenges persist, the integration of emerging technologies and multidisciplinary efforts will drive the field forward, unlocking new possibilities for health and scientific breakthroughs.
References
[1] Ganguly S, Wakchaure R. Hybridoma technology: a brief review on its diagnostic and clinical significance [J]. Pharmaceut Biol Eval. 2016;3(issue 6):554–555.
[2] Parray HA, Shukla S, Samal S, et al. Hybridoma technology a versatile method for isolation of monoclonal antibodies, its applicability across species, limitations, advancement and future perspectives [J]. Int Immunopharmacol. 2020;85:106639.
[3] Bodeus M, Burtonboy G, Bazin H. Rat monoclonal antibodies. IV. Easy method for In-vitro production. J Immunol Method [J]. 1985;79:1–6.
[4] Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity [J]. Nature. 1975;256(5517):495–497.
CUSABIO team. A Comprehensive Guide to Monoclonal Antibody Production. https://www.cusabio.com/c-21200.html



Comments
Leave a Comment