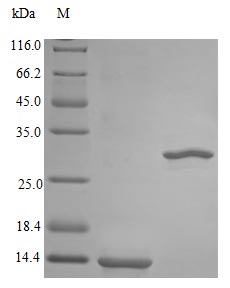
Recombinant Rhesus Macaque Interleukin-5 protein (IL5) is produced in E. coli and comes without any tags, which should minimize interference with its biological function. The protein represents the full mature form, covering amino acids 20-134. Testing shows purity levels above 98% based on SDS-PAGE analysis. Biological activity appears robust, with an ED50 below 4 ng/ml when tested in human TF-1 cell proliferation assays. This translates to a specific activity exceeding 2.5 × 10^5 IU/mg. Endotoxin contamination remains low at less than 1.0 EU/µg, as measured by the LAL method.
Interleukin-5 (IL5) is a cytokine that seems particularly important for eosinophil regulation and activation. Eosinophils are specialized white blood cells that respond to immune challenges and allergic reactions. IL5 appears central to both the growth and differentiation of these cells. This makes it valuable for researchers studying immune system mechanisms and related disorders. The protein's role in inflammation and immune regulation pathways may explain why it draws significant scientific interest.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
1. Cell Proliferation and Viability Assays for Eosinophil Research
This recombinant rhesus macaque IL-5 is confirmed to be biologically active (ED₅₀ < 4 ng/ml in human TF-1 cells) and suitable for eosinophil studies. However, researchers should validate its activity in rhesus macaque eosinophil systems to confirm that cross-species reactivity is consistent. The high purity (>98%) supports reliable dose-response studies, but optimal concentrations may need adjustment for primary eosinophil cultures where receptor expression and signaling may differ from TF-1 cells.
2. Cross-Species Cytokine Activity Studies
The demonstrated activity in human TF-1 cells enables valid cross-species comparisons, but researchers should note that IL-5 receptor affinity may vary between species. Comparative studies should include parallel assays in both human and rhesus systems to properly interpret functional conservation. The high specific activity (>2.5×10⁵ IU/mg) provides a quantitative basis for potency comparisons.
3. Antibody Development and Validation
This high-purity, full-length mature IL-5 (20-134aa) serves as an excellent antigen for antibody development. However, antibodies should be validated against native rhesus IL-5 from biological sources to ensure recognition of physiologically relevant forms. The confirmed cross-species bioactivity indicates proper folding of conformational epitopes.
4. Biochemical Characterization and Protein-Protein Interaction Studies
The biologically active IL-5 is suitable for IL-5Rα binding studies, but the E. coli expression produces a non-glycosylated protein, which may affect some receptor interactions. Binding assays should validate that kinetics match mammalian-expressed IL-5. The tag-free design ensures authentic interactions, but researchers should confirm dimerization properties as IL-5 functions as a homodimer.
5. Preclinical Disease Model Development
This rhesus-specific IL-5 is appropriate for preclinical models, but researchers should account for the potential immunogenicity of the human sequence in primate studies. The protein's activity in human cells supports translational relevance, but in vivo studies should include appropriate controls for species-specific responses and potential neutralizing antibodies in chronic models.
Final Recommendation & Action Plan
This recombinant rhesus macaque IL-5 is a high-quality reagent with demonstrated cross-species bioactivity, making it suitable for all proposed applications with appropriate validation for species-specific effects. For immediate use, employ it at 1-10 ng/ml based on the ED₅₀, but establish dose-response curves in rhesus-specific primary eosinophil cultures to confirm optimal concentrations for physiological studies. When developing antibodies, this full-length protein is ideal for comprehensive epitope coverage, but validates cross-reactivity with native rhesus IL-5 from stimulated T-cells or biological fluids. For binding studies, the tag-free design ensures authentic receptor interactions, but confirms dimer formation and compares binding kinetics with mammalian-expressed IL-5 to account for potential glycosylation effects. The E. coli expression produces a non-glycosylated protein, which is acceptable as IL-5 has minimal glycosylation, but critical findings should be verified in physiologically relevant systems. For preclinical models, the rhesus-specific sequence minimizes immunogenicity concerns, but monitor for antibody development in long-term studies. Always include proper controls and consider that different eosinophil sources (bone marrow vs peripheral blood) may exhibit varying sensitivity to IL-5 stimulation.