[1] Yu, Lei, et al. "HSF1 promotes CD69+ Treg differentiation to inhibit colitis progression." Theranostics 13.6 (2023): 1892.
[2] Dohnálek, Jan, and Tereza Skálová. "C-type lectin-(like) fold-protein-protein interaction patterns and utilization." Biotechnology Advances 58 (2022): 107944.
[3] Koyama-Nasu, Ryo, et al. "The cellular and molecular basis of CD69 function in anti-tumor immunity." International Immunology 34.11 (2022): 555-561.
[4] Vandeveer, George H., et al. "Discovery of structurally diverse reversible BTK inhibitors utilized to develop a novel in vivo CD69 and CD86 PK/PD mouse model." Bioorganic & Medicinal Chemistry Letters 80 (2023): 129108.
[5] Lauzurica, Pilar, et al. "Phenotypic and functional characteristics of hematopoietic cell lineages in CD69-deficient mice." Blood, The Journal of the American Society of Hematology 95.7 (2000): 2312-2320.
[6] Chen, Chun-Kai, et al. "Increased expressions of CD69 and HLA-DR but not of CD25 or CD71 on endometrial T lymphocytes of nonpregnant women." Human immunology 42.3 (1995): 227-232.
[7] Peggs, Karl S., et al. "Immunotherapy with CD25/CD71-allodepleted T cells to improve T-cell reconstitution after matched unrelated donor hematopoietic stem cell transplant: a randomized trial." Cytotherapy 25.1 (2023): 82-93.
[8] Cibrián, Danay, and Francisco Sánchez-Madrid. "CD69: from activation marker to metabolic gatekeeper. "European journal of immunology 47.6 (2017): 946-953.
[9] Agaugué, Sophie, et al. "Human natural killer cells exposed to IL-2, IL-12, IL-18, or IL-4 differently modulate priming of naive T cells by monocyte- derived dendritic cells." Blood, The Journal of the American Society of Hematology 112.5 (2008): 1776-1783.
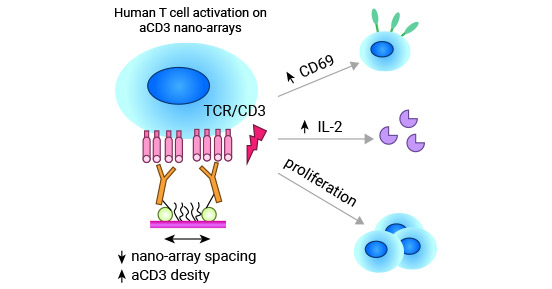
[10] Matic, J., Deeg, J., Scheffold, A., Goldstein, I., & Spatz, J. P. (2013). Fine tuning and efficient T cell activation with stimulatory aCD3 nanoarrays. nano letters, 13(11), 5090-5097.
[11] Tian, Yunfei, et al. "Development of a Monoclonal Antibody to Pig CD69 Reveals Early Activation of T Cells in Pig after PRRSV and ASFV Infection." Viruses 14.6 (2022): 1343.
[12] Borrego, F., et al. "CD69 is a stimulatory receptor for natural killer cell and its cytotoxic effect is blocked by CD94 inhibitory receptor." Immunology 97.1 (1999): 159.
[13] Zingoni, Alessandra, et al. "CD69-triggered ERK activation and functions are negatively regulated by CD94/NKG2-A inhibitory receptor." European journal of immunology 30.2 (2000): 644-651.
[14] Ishizaki, Shunsuke, et al. "Role of CD69 in acute lung injury." life sciences 90.17-18 (2012): 657-665.
[15] Lamana, Amalia, et al. "CD69 modulates sphingosine-1-phosphate-induced migration of skin dendritic cells." Journal of Investigative Dermatology 131.7 (2011): 1503-1512.
[16] Ramı́rez, Rafael, et al. "CD69-induced monocyte apoptosis involves multiple nonredundant signaling pathways. "Cellular immunology 172.2 (1996). 192-199.
[17] Aouad, Salah M., et al. "Caspase-3 is a component of Fas death-inducing signaling complex in lipid rafts and its activity is required for complete caspase-8 activation during Fas-mediated cell death." The Journal of Immunology 172.4 (2004): 2316-2323. -8 activation during Fas-mediated cell death." The Journal of Immunology 172.4 (2004): 2316-2323.
[18] Deng, Caishu, Elzbieta Goluszko, and Premkumar Christadoss. "Fas/Fas ligand pathway, apoptosis, and clonal anergy involved in systemic acetylcholine receptor T cell epitope tolerance." The Journal of Immunology 166.5 (2001): 3458-3467.
[19] Qiu, Guo, Xi Xu, and Qifa Liu. "CD69 Marks Leukemic Regenerating Cells and Regulates Their Metabolism in AML." Blood 140.Supplement 1 (2022): 8780-8782.
[20] Del Poeta, Giovanni, et al. "CD69 is independently prognostic in chronic lymphocytic leukemia: a comprehensive clinical and biological profiling study." Haematologica 97.2 (2012): 279-287.
[21] Davison, Glenda M., Nicolas Novitzky, and Rygana Abdulla. "Monocyte derived dendritic cells have reduced expression of co-stimulatory molecules but are able to stimulate autologous T-cells in patients with MDS." Hematology/oncology and stem cell therapy 6.2 (2013): 49-57.
[22] Portales-Perez, D., et al. "Abnormalities in CD69 expression, cytosolic pH and Ca2+ during activation of lymphocytes from patients with systemic lupus erythematosus." Lupus 6.1 (1997): 48-56.
[23] Atzeni, Fabiola, et al. "CD69 expression on neutrophils from patients with rheumatoid arthritis." clinical and experimental rheumatology 22.3 (2004) : 331-334.
[24] Afeltra, A., et al. "Expression of CD69 antigen on synovial fluid T cells in patients with rheumatoid arthritis and other chronic synovitis." Annals of the rheumatic diseases 52.6 (1993): 457-460.
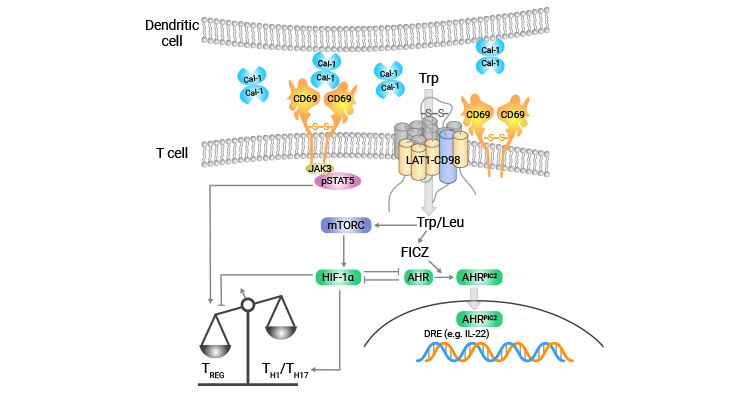
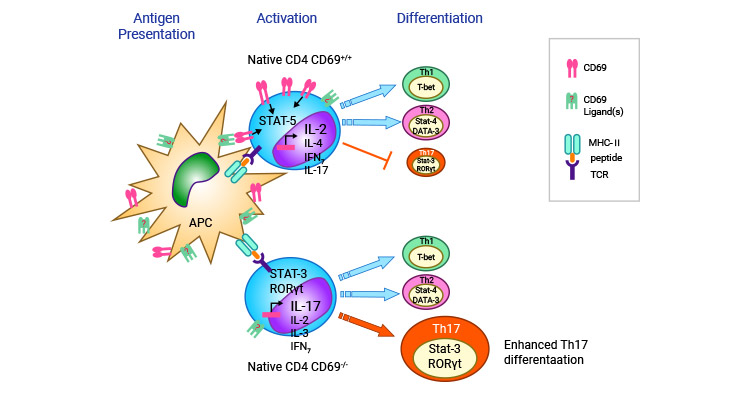
[25] Martín, Pilar, et al. "CD69 association with Jak3/Stat5 proteins regulates Th17 cell differentiation." Molecular and cellular biology 30.20 (2010). 4877-4889.
[26] Ivanovska, Nina, and Petya Dimitrova. "Bone resorption and remodeling in murine collagenase-induced osteoarthritis after administration of glucosamine." Arthritis research & therapy 13.2 (2011): 1-13.
[27] Peng, Jianqiao, and Yi Xiang. "Value analysis of CD69 combined with EGR1 in the diagnosis of coronary heart disease. "Experimental and Therapeutic Medicine 17.3 (2019): 2047-2052.
[28] Martín, Pilar, et al. "The leukocyte activation antigen CD69 limits allergic asthma and skin contact hypersensitivity." Journal of Allergy and Clinical Immunology 126.2 (2010): 355-365.
[29] Cai, Yang, Wei Zhu, and Guanghua Feng. "Alteration of CD69 and HLA-DR-positive T Cells in Patients with Gastric Cancer after Operations and its Clinical Significances." Journal of Medical Research (2006).
[30] Bruni, Elena, et al. "Intrahepatic CD69+ Vδ1 T cells re-circulate in the blood of patients with metastatic colorectal cancer and limit tumor progression ." Journal for Immunotherapy of Cancer 10.7 (2022).
[31] Tang, Kaihua, et al. "CD69 serves as a potential diagnostic and prognostic biomarker for hepatocellular carcinoma." Scientific Reports 13.1 (2023). 7452.
[32] Montraveta, Arnau, et al. "CD69 expression potentially predicts response to bendamustine and its modulation by ibrutinib or idelalisib enhances cytotoxic effect in chronic lymphocytic leukemia." Oncotarget, 2015, vol. 7, num. 5, p. 5507-5520 (2015).
[33] Chen, Hongwen, et al. "Transmembrane protein CD69 acts as an S1PR1 agonist." Elife 12 (2023): e88204.

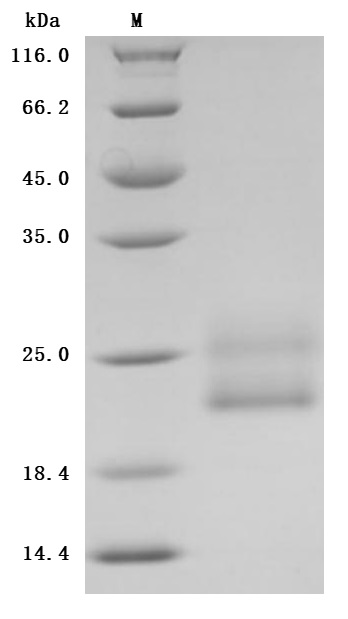
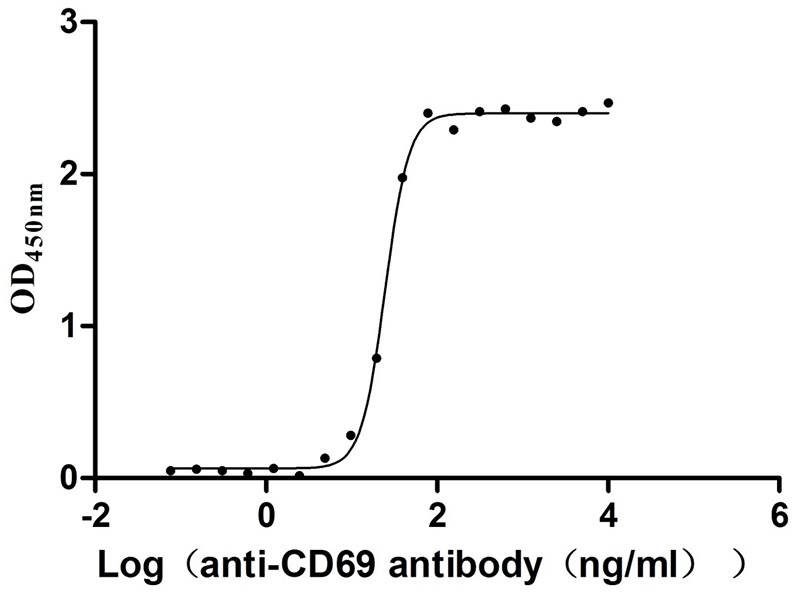
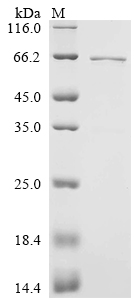
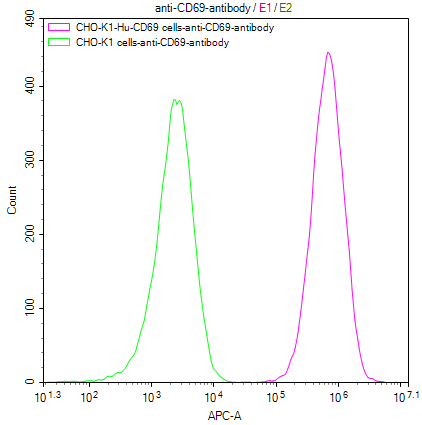


Comments
Leave a Comment