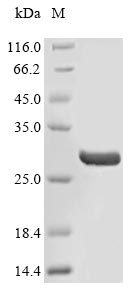
The recombinant human Fibronectin (FN1) protein expression in E.coli cells requires the insertion of a DNA fragment encoding the human FN1 protein (732-911aa) into a plasmid vector along with an N-terminal His tag gene and the transferral of this vector into E.coli cells. The positive cells are screened, cultured, and induced to express the FN1 protein. The cells are lysed to harvest the recombinant human FN1 protein, which is purified through affinity purification and then detected by SDS-PAGE and subsequent staining of the gel with Coomassie Brilliant Blue. The purity of this recombinant human FN1 protein is over 90%.
FN1 is a large glycoprotein that plays a significant role in the extracellular matrix (ECM) assembly. It acts as a scaffold for depositing other ECM proteins, such as collagens [2]. Fibronectin facilitates interactions between cells and the ECM [1]. It binds to integrins via specific binding sites, notably the Arg-Gly-Asp (RGD) sequence, promoting the formation of insoluble fibrils within the ECM [3]. Integrins, such as αVβ1 and α5β1, interact with fibronectin and regulate key cellular processes including embryogenesis, wound healing, blood coagulation, host defense, and metastasis [4][5]. The matrix form of fibronectin is essential for cell adhesion and migration, particularly during embryogenesis, wound healing, and inflammatory responses [6].
References:
[1] V. Koteliansky, M. Glukhova, M. Benjamin, В. Смирнов, V. Filimonov, O. Zaliteet al., A study of the structure of fibronectin, European Journal of Biochemistry, vol. 119, no. 3, p. 619-624, 1981. https://doi.org/10.1111/j.1432-1033.1981.tb05652.x
[2] C. Miller, G. Budoff, J. Prenner, & J. Schwarzbauer, Minireview: fibronectin in retinal disease, Experimental Biology and Medicine, vol. 242, no. 1, p. 1-7, 2016. https://doi.org/10.1177/1535370216675245
[3] P. Koistinen and J. Heino, The selective regulation of αvβ1 integrin expression is based on the hierarchical formation of αv-containing heterodimers, Journal of Biological Chemistry, vol. 277, no. 27, p. 24835-24841, 2002. https://doi.org/10.1074/jbc.m203149200
[4] S. Blystone, I. Graham, F. Lindberg, & E. Brown, Integrin alpha v beta 3 differentially regulates adhesive and phagocytic functions of the fibronectin receptor alpha 5 beta 1., The Journal of Cell Biology, vol. 127, no. 4, p. 1129-1137, 1994. https://doi.org/10.1083/jcb.127.4.1129
[5] Q. Zhang, T. Sakai, J. Nowlen, I. Hayashi, R. Fässler, & D. Mosher, Functional β1-integrins release the suppression of fibronectin matrix assembly by vitronectin, Journal of Biological Chemistry, vol. 274, no. 1, p. 368-375, 1999. https://doi.org/10.1074/jbc.274.1.368
[6] J. Hang, E. Zemskov, L. Lóránd, & A. Belkin, Identification of a novel recognition sequence for fibronectin within the nh2-terminal β-sandwich domain of tissue transglutaminase, Journal of Biological Chemistry, vol. 280, no. 25, p. 23675-23683, 2005. https://doi.org/10.1074/jbc.m503323200