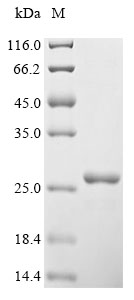
To prepare the recombinant human VEGFA protein with an N-terminal 6xHis tag, the VEGFA gene (27-232aa) is co-cloned into a plasmid with the tag gene. The constructed plasmids are transfected into yeast cells, which are induced to express the target protein after adding IPTG. The cells are lysed to release the recombinant VEGFA protein, which is purified using Ni-NTA affinity chromatography, where the His tag allows for selective binding to the nickel matrix. Following elution, SDS-PAGE analysis shows the protein to be highly pure, with a purity exceeding 90%.
Human VEGFA is a critical cytokine particularly involved in angiogenesis, which is the formation of new blood vessels from pre-existing ones. VEGFA plays a fundamental role in embryonic development, wound healing, and the pathogenesis of several diseases, including cancer and cardiovascular disorders.
VEGFA is primarily produced by endothelial cells. Its expression is regulated by hypoxia and other stimuli, which enhance its production to promote angiogenesis in response to tissue oxygen demands [1][2]. The signaling pathway of VEGFA is mediated through its interaction with two main receptors: VEGFR1 (Flt-1) and VEGFR2 (KDR), which are expressed on endothelial cells. This interaction triggers a cascade of intracellular signaling that leads to endothelial cell proliferation, migration, and increased vascular permeability [3][4].
VEGFA is often overexpressed in cancer, facilitating tumor growth and metastasis by promoting neovascularization. This is particularly evident in solid tumors, where the demand for oxygen and nutrients increases as the tumor mass expands [5][6]. Elevated levels of VEGFA in the serum have been associated with poor prognosis in various malignancies, including breast and prostate cancer [7][8]. Moreover, VEGFA's role in enhancing vascular permeability contributes to the formation of ascites in ovarian cancer, highlighting its importance in tumor microenvironment dynamics [9].
VEGFA is crucial in reproductive biology, particularly in trophoblast development during pregnancy. It is produced by both trophoblasts and the endometrium, playing a vital role in placental angiogenesis and the establishment of maternal-fetal circulation [10][11]. Abnormal levels of VEGFA during pregnancy can lead to complications such as preeclampsia, underscoring its significance in vascular health [11].
References:
[1] X. Hu, W. Yang, L. Du, W. Cui, W. Mi, Q. Mao-Ying, et al., Vascular endothelial growth factor a signaling promotes spinal central sensitization and pain-related behaviors in female rats with bone cancer, Anesthesiology, vol. 131, no. 5, p. 1125-1147, 2019. https://doi.org/10.1097/aln.0000000000002916
[2] A. Wang, D. Leong, Z. He, L. Xu, L. Liu, D. Hirsh, et al. Procyanidins mitigate osteoarthritis pathogenesis by, at least in part, suppressing vascular endothelial growth factor signaling, International Journal of Molecular Sciences, vol. 17, no. 12, p. 2065, 2016. https://doi.org/10.3390/ijms17122065
[3] I. Gisterek, R. Matkowski, A. Łacko, P. Sedlaczek, K. Szewczyk, P. Biecek, et al. Serum vascular endothelial growth factors a, c and d in human breast tumors, Pathology & Oncology Research, vol. 16, no. 3, p. 337-344, 2009. https://doi.org/10.1007/s12253-009-9211-8
[4] M. Shibuya, Vascular endothelial growth factor receptor-1 (vegfr-1/flt-1): a dual regulator for angiogenesis, Angiogenesis, vol. 9, no. 4, p. 225-230, 2006. https://doi.org/10.1007/s10456-006-9055-8
[5] H. Li, P. Kantoff, J. Ma, M. Stampfer, & D. George, Prediagnostic plasma vascular endothelial growth factor levels and risk of prostate cancer, Cancer Epidemiology Biomarkers & Prevention, vol. 14, no. 6, p. 1557-1561, 2005. https://doi.org/10.1158/1055-9965.epi-04-0456
[6] , The supression of migration and metastasis via inhibition of vascular endothelial growth factor in pancreatic adenocarcinoma cells applied danusertib, Turkish Journal of Gastroenterology, vol. 35, no. 2, p. 150-157, 2024. https://doi.org/10.5152/tjg.2024.22319
[7] J. Duque, K. Loughlin, R. Adam, P. Kantoff, E. Mazzucchi, & M. Freeman, Measurement of plasma levels of vascular endothelial growth factor in prostate cancer patients: relationship with clinical stage, gleason score, prostate volume, and serum prostate-specific antigen, Clinics, vol. 61, no. 5, p. 401-408, 2006. https://doi.org/10.1590/s1807-59322006000500006
[8] A. Mitsuhashi, K. Suzuka, K. Yamazawa, H. Matsui, K. Seki, & S. Sekiya, Serum vascular endothelial growth factor (vegf) and vegf‐c levels as tumor markers in patients with cervical carcinoma, Cancer, vol. 103, no. 4, p. 724-730, 2005. https://doi.org/10.1002/cncr.20819
[9] L. Hu, N. Ferrara, & R. Jaffe, Paracrine vegf/ve-cadherin action on ovarian cancer permeability, Experimental Biology and Medicine, vol. 231, no. 10, p. 1646-1652, 2006. https://doi.org/10.1177/153537020623101010
[10] F. Cabar, P. Pereira, M. Oliveira, & R. Francisco, Serum vascular endothelial growth factor as a marker for tubal pregnancy, Revista Da Associação Médica Brasileira, vol. 68, no. 6, p. 860-865, 2022. https://doi.org/10.1590/1806-9282.20220224
[11] A. Bogacz, P. Mikołajczak, M. Wolek, A. Górska, M. Szulc, M. Ożarowski, et al., Combined effects of methyldopa and flavonoids on the expression of selected factors related to inflammatory processes and vascular diseases in human placenta cells—an in vitro study, Molecules, vol. 26, no. 5, p. 1259, 2021. https://doi.org/10.3390/molecules26051259