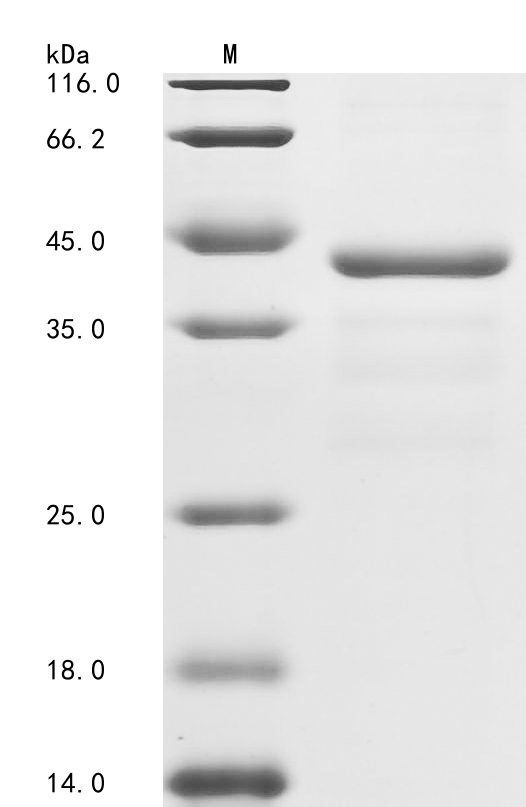
The generation of the recombinant human coagulation factor IX (F9) protein begins with the co-cloning of the coagulation factor IX gene fragment (144-239aa) with the N-terminal 6xHis-GST tag gene into a vector. The constructed vectors are transformed into E.coli cells, which are induced with IPTG to express the recombinant protein. After cell lysis, Ni-NTA affinity chromatography is employed to purify the protein by exploiting the affinity between the 6xHis tag and nickel ions. The purified coagulation factor IX protein is then subjected to SDS-PAGE analysis to assess its purity, which consistently exceeds 85%.
Human coagulation factor IX (FIX/F9) is a crucial vitamin K-dependent plasma protein that plays a significant role in the intrinsic pathway of blood coagulation. It is primarily synthesized in the liver and is essential for the conversion of factor X to its active form FXa, in the presence of calcium ions and phospholipids, which is a critical step in the coagulation cascade [1]. Deficiencies or mutations in the FIX gene lead to hemophilia B, an X-linked bleeding disorder characterized by recurrent spontaneous bleeding episodes [2][3].
The structure of FIX consists of a light chain and a heavy chain, connected by a linker region, which is vital for its function [4]. The activity of FIX is tightly regulated, and its deficiency can result in severe clinical manifestations, including hemarthrosis and internal bleeding, which can be life-threatening [5]. Recent advancements in gene therapy have shown promise for treating hemophilia B by restoring FIX levels. For instance, studies utilizing CRISPR/Cas9 technology have successfully knocked in human FIX into the swine F9 locus, demonstrating significant therapeutic effects in hemophilia B models [1].
References:
[1] J. Chen, B. An, B. Yu, X. Peng, H. Yuan, Q. Yang, et al. Crispr/cas9-mediated knockin of human factor ix into swine factor ix locus effectively alleviates bleeding in hemophilia b pigs, Haematologica, vol. 106, no. 3, p. 829-837, 2020. https://doi.org/10.3324/haematol.2019.224063
[2] R. Nigam, R. Choudhary, R. Malik, S. Kothari, K. Verma, A. Shrivastavae, et al. Clinicohematological study of hemophilia patients in bhopal, Journal of Evolution of Medical and Dental Sciences, vol. 3, no. 11, p. 2910-2916, 2014. https://doi.org/10.14260/jemds/2014/2224
[3] L. Ramos-Petersen, A qualitative study exploring the experiences and perceptions of patients with hemophilia regarding their health-related well-being, in salamanca, Journal of Clinical Medicine, vol. 12, no. 16, p. 5417, 2023. https://doi.org/10.3390/jcm12165417
[4] H. Kitano, A. Mamiya, T. Ishikawa, S. Kokubun, & C. Hidai, Coagulation factor ix regulates cell migration and adhesion in vitro, Cell Biology International, vol. 39, no. 10, p. 1162-1172, 2015. https://doi.org/10.1002/cbin.10491
[5] K. Ohashi, K. Tatsumi, R. Utoh, S. Takagi, M. Shima, & T. Okano, Engineering liver tissues under the kidney capsule site provides therapeutic effects to hemophilia b mice, Cell Transplantation, vol. 19, no. 6-7, p. 807-813, 2010. https://doi.org/10.3727/096368910x508924