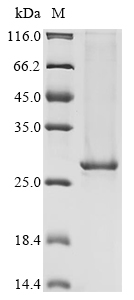
This recombinant human Tyrosine-protein kinase BTK comes from an E. coli expression system and covers amino acids 396-659. The protein appears to maintain its native structure and function since it's expressed without tags. SDS-PAGE analysis shows purity levels above 90%. This product is strictly for research purposes—not for diagnostic or therapeutic use.
BTK (Bruton's tyrosine kinase) seems to be central to B-cell receptor signaling and is likely critical for proper B-cell development and function. The protein participates in various signaling cascades that may control cell growth, differentiation, and programmed cell death. For immunology researchers, BTK represents an important tool for understanding immune responses and exploring potential therapeutic targets.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
This fragment likely contains the kinase domain, but BTK requires correct folding, phosphorylation (e.g., at Tyr551 in the activation loop), and potential interactions with other domains for bioactivity. E. coli lacks eukaryotic chaperones and post-translational modification machinery, making proper folding and activation unlikely. Without experimental validation (e.g., kinase assays or structural analysis), the protein is probably misfolded and inactive. The high purity indicates low impurities, but does not ensure native conformation or function.
1. In Vitro Protein-Protein Interaction Studies
This application is not feasible without affinity tags for immobilization, as the lack of tags (e.g., His-tag) makes pull-down or co-immunoprecipitation experiments challenging. Even if alternative methods are used, misfolding could lead to non-physiological interactions. If interactions are studied, validate any findings with full-length, properly folded BTK from eukaryotic systems to ensure relevance.
2. Antibody Development and Validation
This recombinant BTK fragment can be used as an immunogen for antibody production, as antibodies may recognize linear epitopes even in misfolded proteins. The high purity supports consistent immunization. However, antibodies generated might not bind to native, full-length BTK in cells due to conformational epitope differences. Validate antibody specificity against full-length BTK expressed in mammalian cells for applications like Western blot or immunofluorescence.
3. Biochemical Characterization and Stability Studies
Suitable for basic biophysical analyses (e.g., circular dichroism for secondary structure, dynamic light scattering for aggregation state). However, data may not reflect the native kinase domain's properties due to potential misfolding. Avoid extrapolating to functional mechanisms without activity validation. Use the protein for stability studies, but interpret results in the context of the recombinant fragment.
4. Competitive Binding Assays and Inhibitor Screening
This application requires a bioactive kinase domain for meaningful results. Without confirmed kinase activity, binding assays will not reliably identify inhibitors. First, validate activity using a kinase assay (e.g., with substrates like ATP). If inactive, do not use for screening; instead, use a properly folded and activated BTK kinase domain.
Final Recommendation & Action Plan
Before using this recombinant BTK fragment for any functional application, prioritize experimental validation of its folding and bioactivity. Start with biophysical assays (e.g., circular dichroism to check for expected α-helical and β-sheet content typical of kinases) and a kinase activity assay using a synthetic substrate or autophosphorylation test. If activity is confirmed, proceed with inhibitor screening or binding studies, but use appropriate controls (e.g., known BTK inhibitors). For antibody development, proceed but validate antibodies against native BTK. Given the lack of tags, avoid interaction studies without first introducing a tag for immobilization or using alternative methods like surface plasmon resonance with tagged versions. For reliable results, consider expressing the kinase domain in a eukaryotic system (e.g., insect cells) that supports proper folding and phosphorylation.