Protein phosphorylation, the reversible addition of a phosphate group to specific amino acid residues in proteins, is a fundamental post-translational modification (PTM) that regulates numerous cellular processes. This essay provides a comprehensive overview of protein phosphorylation, including its mechanism, enzymes involved, and the profound impact it has on protein structure, function, and cellular signaling pathways.
Furthermore, it explores the diverse roles of protein phosphorylation in key biological processes such as signal transduction, cell cycle regulation, and gene expression. It will explore the diverse roles of protein phosphorylation in signal transduction, cell cycle regulation, gene expression, and cellular responses. Also, it will discuss the dysregulation of protein phosphorylation in diseases and the potential for targeting protein phosphorylation for therapeutic interventions.
1. What Is Protein Phosphorylation?
Protein phosphorylation, the covalent addition of a phosphate group to specific amino acid residues, is a key regulatory mechanism in cellular signaling and control. It enables cells to rapidly respond to environmental cues and regulate a wide range of biological processes.
2. Phosphorylation Mechanism
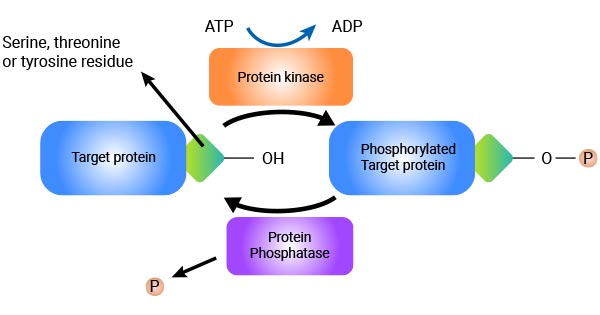
Protein phosphorylation involves the transfer of a phosphate group from ATP to specific amino acid residues in proteins [1]. The most common phosphorylation sites are serine, threonine, and tyrosine residues, although other residues such as histidine and aspartate can also be phosphorylated. This process is catalyzed by enzymes called kinases, which transfer the phosphate group to the target protein. On the contrary, protein phosphatases catalyze the removal of phosphate groups from phosphorylated proteins, thereby reversing the phosphorylation process.
Figure 1: Protein Phosphorylation mechanism
Phosphorylation serves as a key mechanism for activating protein kinases, initiating a cascade of events that culminate in the phosphorylation of different amino acids [4]. The activation or deactivation of kinases can occur through multiple mechanisms, including cis-phosphorylation/autophosphorylation within the kinase itself and binding with activator or inhibitor proteins or checking their localization in the cell in relation to their substrate [5].
It is estimated that up to 30% of all human proteins undergo modification through kinase activity, highlighting the extensive reach and influence of kinases in regulating cellular pathways. Notably, kinases play a crucial role in modulating signal transduction, governing the majority of cellular pathways involved in cellular communication and response [6].
Protein kinases are a diverse group of enzymes that catalyze the phosphorylation of target proteins. Kinases play a pivotal role in cellular signaling by regulating protein activity, protein-protein interactions, and the initiation of downstream signaling cascades. They are classified into several families according to the amino acid residue that it phosphorylates.
Here are some of the major classes of protein kinases:
|
Classification
|
Members
|
Function
|
|
Serine/Threonine Protein Kinases (STKs)
|
AGC
|
PKA, PKG, PKC, Akt/PKB, S6 Kinase (S6K), RSK (Ribosomal S6 Kinase), MSPK1, MSPK2, PDK1, PKD, GRK1, GRK2, GRK3, GRK4, GRK5, GRK6, GRK7, GRK8
|
Involved in diverse cellular processes, including metabolism, cell cycle regulation, and neuronal signaling.
|
|
Cyclin-dependent kinases (CDKs)
|
CDK1, CDK2, CDK3, CDK4, CDK5, CDK6, CDK7, CDK8, CDK9, CDK10, CDK11, CDK11A, CDK11B, CDK12, CDK13, CDK14, CDK15, CDK16, CDK17, CDK18, CDK19, CDK20
|
CDKs play a central role in the cell cycle regulation network, and its main biological role is to regulate different phases of the cell cycle, from G1, S, G2 to M phase, and complete the cycle. They are also involved in the regulation of transcription, mRNA processing and differentiation of neural cells.
|
|
CAMK
|
CAMK1, CAMK2A, CAMK2B, CAMK2G, CAMK2D, CAMK4, CAMK1L1/MARK1, CAMK1L2/ARK5, CAMK1L3/MARK2, CAMK1L4/SNRK, CAMK1L5/MARK3, CAMK1L6/ MARK4
|
CAMKs are regulated by the binding of Ca2+ and calmodulin and play important roles in neuronal signaling, muscle contraction, and synaptic plasticity.
|
|
CK1 (casein kinase 1)
|
CK1α/CSNK1A1, CK1β/CSNK1B, CK1γ/CSNK1G1, CK1δ/ CSNK1D, CK1ε/CSNK1E, CK1γ/CSNK1G1, CK1ε/CSNK1E
|
Involved in various cellular processes, including circadian rhythm regulation, Wnt signaling, vesicular trafficking, and DNA repair.
|
|
MAPK
|
ERK1/MAPK3, ERK2/MAPK1, ERK3/MAPK6, ERK4/MAPK4, ERK5/MAPK7, JNK1/MAPK8, JNK2/MAPK9, JNK3/MAPK10, p38α/MAPK14, p38β/MAPK11, p38γ/MAPK12, p38δ/MAPK13
|
Key regulators of cellular responses to extracellular stimuli, such as growth factors, stress, and cytokines. They are involved in various biological processes, including cell proliferation, differentiation, survival, stress response, and immune regulation.
|
|
Tyrosine Protein Kinases (TKs)
|
Receptor Tyrosine Kinases (RTKs)
|
EGFR/ErbB1/HER1, ErbB2/HER2, ErbB3/HER3, ErbB4/HER4, insulin receptor (IR), IGF1R, FGFR1, FGFR2, FGFR3, FGFR4, PDGFRA, PDGFRB, VEGFR1/Flt1, VEGFR2/KDR/Flk1, VEGFR3 /Flt4, HGFR, KIT/CD117, RET, EphA1, EphA2, EphA3, EphA4, EphA5, EphB1, EphB2, EphB3, EphB4, EphB6, ALK
|
RTKs play crucial roles in cell growth, differentiation, and survival. They are activated by ligand binding and include receptors for growth factors, cytokines, and hormones.
|
|
Non-receptor Tyrosine Kinases (NRTKs)
|
Src, Yes, Fyn, Lck, Lyn, Blk, Hck, Abl1, Abl2, Tec, Btk, Itk, Bmx, JAK1, JAK2, JAK3, Tyk2, Syk, ZAP70, Fer, Fes
|
Involved in intracellular signaling pathways and regulate processes such as cell proliferation, migration, and immune responses.
|
|
Dual-Specificity Kinases
|
MAPK Kinases (MAPKKs) or MEKs
|
MAP2K1/MEK1, MAP2K2/MEK2, MAP2K3/MKK3, MAP2K4/MKK4, MAP2K5/MKK5, MAP2K6/MKK6, MAP2K7/MKK7, MAP2K8/MKK8, MAP2K9/MKK9, MAP2K10/MKK10, MAP2K11/MKK11
|
MAPKKs phosphorylate and activate MAPKs, which in turn regulate various cellular processes, including cell proliferation, differentiation, apoptosis, and stress responses.
|
|
Dual-Specificity Tyrosine-Regulated Kinases (DYRKs)
|
DYRK1A, DYRK1B, DYRK2, DYRK3, DYRK4
|
DYRKs phosphorylate both serine/threonine and tyrosine residues and are involved in various cellular processes, including development, cell cycle regulation, and neuronal function.
|
2.2 Protein Phosphatases
Phosphatases perform the opposite function of kinases. They act as "molecular scissors,"cleaving phosphoric acid monoesters into a phosphate group and a molecule with a free hydroxyl group [7][8], thus reversing the phosphorylation process.
Phosphatases play crucial roles in terminating signaling events, resetting protein activity, and maintaining cellular homeostasis. They are classified into several families based on their catalytic subunits and substrate specificity. Here are the major classes of protein phosphatases and their corresponding functions:
|
Classification
|
Function
|
|
Protein Tyrosine Phosphatases (PTPs)
|
Receptor-type PTPs
|
PTPRC/CD45, PTPRF, PTPRJ, PTPRO, PTPRT
|
PTPs dephosphorylate tyrosine residues in proteins. They play important roles in cellular signaling, including receptor tyrosine kinase signaling, modulation of growth factor signaling and cell adhesion, and control of cell cycle progression. PTPs are involved in the regulation of diverse cellular processes, such as cell growth, differentiation, and immune response.
|
|
Non-receptor-type PTPs
|
PTPN1/PTP1B, PTPN2/TCPTP, PTPN11/SHP2, DUSP3/VHR
|
|
Serine/Threonine Phosphatases (STPs)
|
phosphoprotein phosphatases (PPPs)
|
Protein Phosphatase 1 (PP1), PP2A, PP2B, PP4, PP5, PP6, PP7
|
PPPs regulates cellular processes such as glycogen metabolism, cell cycle progression, gene expression, protein synthesis,
cytoskeletal dynamics. calcium-dependent signaling pathways, immune response, muscle contraction, and neuronal function. PPMs require divalent metal ions, such as magnesium or manganese, for their catalytic activity. They are involved in diverse cellular processes, including cell cycle regulation, DNA damage response, and cellular stress responses.
|
|
metallo-dependent protein phosphatases (PPMs)
|
PPM1A, PPM1X, PP2C
|
|
Dual Specificity Phosphatases (DSPs)
|
DUSP1/MKP-1, DUSP6/MKP-3, VHR/DUSP3
|
DSPs are a class of protein phosphatases that can dephosphorylate both phosphotyrosine and phosphoserine/phosphothreonine residues in target proteins. They regulate various signaling pathways, including MAPK signaling, cell cycle progression, and immune responses.
|
|
Aspartate-Based Phosphatases
|
Haloacid Dehalogenase (HAD) superfamily phosphatases
|
PPM1A, PPM2
|
Aspartate-based phosphatases are a group of protein phosphatases that dephosphorylate aspartate residues in their target proteins. They are involved in the regulation of bacterial two-component signaling systems, which play critical roles in bacterial responses to environmental changes.
|
|
Cysteine-Based Protein Phosphatases (CBPs)
|
CPTP/PTPN22, LMPTP/ACP1
|
They dephosphorylate tyrosine-phosphorylated proteins and are responsible for regulation of cell cycle progression, cellular stress responses, and cellular growth and differentiation and maintenance of protein homeostasis.
|
3. Protein Phosphorylation and Structural Consequences
3.1 Conformational Changes and Protein Dynamics
Phosphorylation can induce conformational changes in proteins, altering their structure and modulating their function [2]. These structural modifications can affect protein-protein interactions, protein stability, subcellular localization, and enzymatic activity. Phosphorylation can act as a molecular switch, turning proteins "on" or "off" in response to various stimuli.
Phosphorylation events can influence protein-protein interactions by creating binding sites for proteins containing specific phospho-recognition domains. This facilitates the assembly of protein complexes and the recruitment of downstream signaling components, thereby amplifying and propagating the signaling cascade.
3.2 Allosteric Regulation
Phosphorylation can regulate protein function through allosteric mechanisms [3]. The addition or removal of a phosphate group at a specific site can induce conformational changes in distal regions of the protein, altering its activity or affinity for ligands. This allosteric regulation allows for fine-tuning of cellular responses and signal amplification.
3.3 Enzyme Activation and Inhibition
Phosphorylation plays a crucial role in regulating enzymatic activity. It can either activate or inhibit enzymes by directly modulating their catalytic sites or by altering their interaction with substrates or cofactors. For example, phosphorylation of serine/threonine kinases can lead to activation, while phosphorylation of tyrosine kinases can result in inhibition.
4. Protein Phosphorylation in Signal Transduction
Phosphorylation acts as a molecular switch, triggering a cascade of events that transmit the signal from the extracellular environment to the nucleus or other cellular compartments, leading to specific cellular responses. It can alter the conformation, activity, subcellular localization, and interaction partners of the phosphorylated proteins, thus modulating their function and downstream signaling.
4.1 RTK signaling Pathway
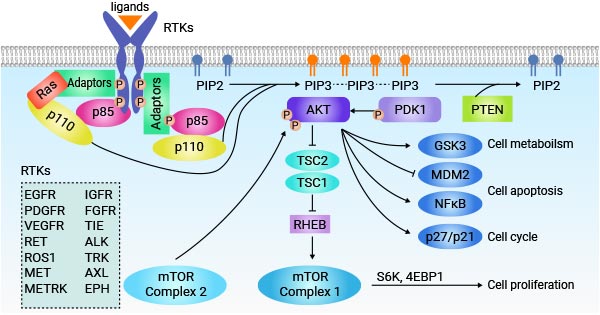
Receptor tyrosine kinases (RTKs) play a vital role in cellular communication by transducing extracellular signals into intracellular responses. Ligand binding to RTKs triggers receptor dimerization and autophosphorylation, leading to the activation of downstream signaling pathways. Phosphorylated tyrosine residues in the receptor act as docking sites for adaptor proteins and downstream effectors, initiating diverse signaling cascades.
Figure 2: RTK signaling pathway
This picture is cited from: https://www.sciencedirect.com/science/article/abs/pii/S1044579X18301172
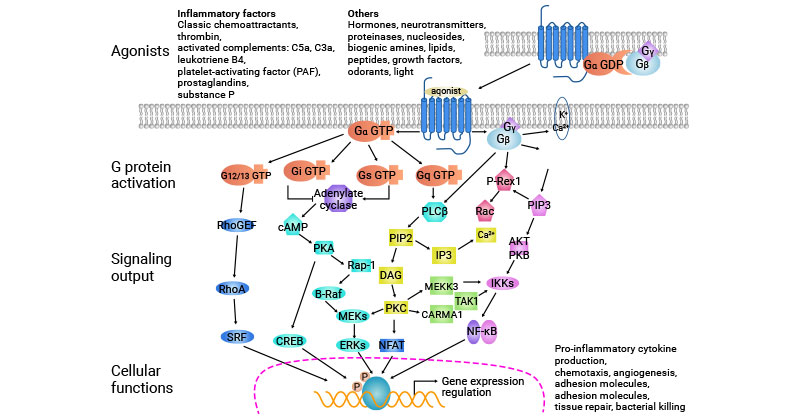
The GPCR signaling pathway starts with ligand binding to the GPCR on the cell surface. This activates the receptor, causing a conformational change. The activated GPCR then interacts with G proteins, leading to the dissociation of the α subunit from the βγ subunits.
The α subunit and βγ subunits independently regulate downstream effectors, initiating intracellular signaling cascades. These cascades generate second messengers that amplify the signal and activate downstream effectors like protein kinases, which ultimately modulate cellular processes, including gene expression, ion channel activity, neurotransmitter release, and cellular metabolism.
Figure 3: GPCR signaling pathway
This picture is cited from: https://www.nature.com/articles/aps2011200
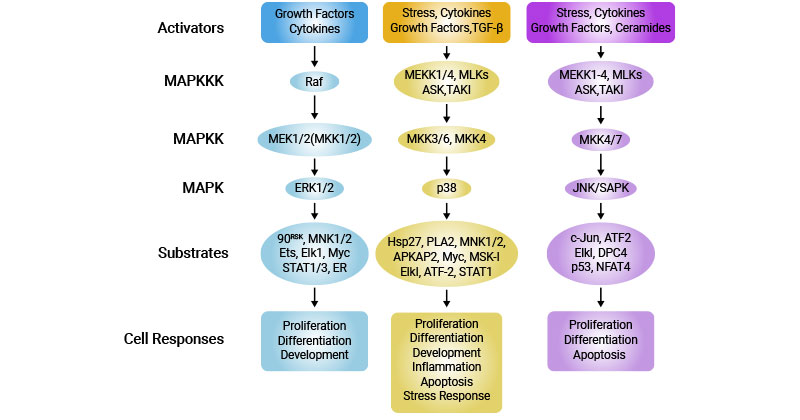
The MAPK pathway is a well-characterized signaling cascade regulated by protein phosphorylation. Activation of MAPKs occurs through sequential phosphorylation events, leading to the activation of downstream effectors such as transcription factors. This pathway is involved in diverse cellular processes, including cell proliferation, differentiation, and response to extracellular signals [9].
Figure 4: Major MAP kinase cascades in mammalian cells
This picture is cited from: https://www.nature.com/articles/7290105
5. Protein Phosphorylation and Cell Cycle Regulation
The cell cycle, a highly regulated process that controls cell division and proliferation, is tightly controlled by protein phosphorylation. Cyclin-dependent kinases (CDKs) and their regulatory subunits, cyclins, orchestrate the progression through different phases of the cell cycle. Phosphorylation of CDKs and their substrates regulates the sequential activation of cell cycle checkpoints and ensures accurate replication and division of genetic material.
5.1 Phosphorylation and Cell Cycle Checkpoints
Phosphorylation plays a crucial role in cell cycle checkpoints, which are surveillance mechanisms that ensure DNA integrity and prevent the propagation of damaged cells. Phosphorylation events at specific checkpoints regulate the activation or inhibition of cell cycle progression, allowing time for DNA repair or triggering programmed cell death if repair is not possible.
5.2 Regulation of Mitosis
Phosphorylation events also regulate mitosis, the process of cell division. Protein phosphorylation orchestrates the assembly and disassembly of the mitotic spindle, chromosome condensation and segregation, and the timing of mitotic entry and exit. Dysregulation of mitotic phosphorylation can lead to abnormal cell division and genomic instability.
6. Protein Phosphorylation and Gene Expression
Protein phosphorylation influences transcriptional regulation, chromatin remodeling, mRNA processing, and stability. Protein phosphorylation acts as a dynamic link between signaling pathways and gene expression programs, ensuring precise and context-dependent control of gene expression patterns in response to cellular and environmental cues.
6.1 Transcription Factors
Phosphorylation regulates the activity of transcription factors, proteins that control gene expression by binding to specific DNA sequences. Phosphorylation events can modulate the DNA-binding affinity, subcellular localization, stability, and transcriptional activity of transcription factors, thus influencing the expression of target genes.
6.2 Chromatin Remodeling
Phosphorylation also regulates chromatin structure and accessibility to transcriptional machinery. Histone proteins, which constitute the core of nucleosomes, can be phosphorylated, leading to alterations in chromatin compaction and the recruitment of chromatin remodeling complexes. These changes facilitate or inhibit gene transcription.
6.3 RNA Polymerase
Regulation Phosphorylation events can regulate the activity of RNA polymerase, the enzyme responsible for synthesizing RNA from a DNA template during transcription. Phosphorylation of the C-terminal domain of RNA polymerase II affects its interaction with transcription factors and the recruitment of other transcriptional machinery, ultimately influencing the transcriptional output.
7. Regulation of Protein Phosphorylation
The regulation of protein phosphorylation involves a complex interplay between kinases, phosphatases, regulatory proteins, and signaling pathways. This dynamic regulation ensures precise control of phosphorylation events, allowing cells to respond to environmental cues, maintain cellular homeostasis, and orchestrate proper physiological processes.
7.1 Kinase Activation and Inhibition
The balance between kinase and phosphatase activity tightly regulates phosphorylation events. Various mechanisms, such as activation or inhibition by other signaling molecules, subcellular localization, and protein-protein interactions, can influence the activity of kinases and phosphatases. Dysregulation of these enzymes can lead to aberrant phosphorylation and contribute to disease pathogenesis.
7.2 Scaffold Proteins and Adapters
Scaffold proteins and adapters play a crucial role in orchestrating phosphorylation events within signaling pathways. They bring together multiple components of the signaling cascade, promoting efficient phosphorylation and facilitating signal propagation. Scaffold proteins also contribute to the specificity and spatial organization of phosphorylation events.
7.3 Crosstalk and Feedback Loops
Phosphorylation events often occur in a context-dependent manner, with crosstalk between different signaling pathways and feedback loops. Crosstalk between pathways enables integration of multiple signals, while feedback loops can fine-tune signaling output and provide robustness to cellular responses. These regulatory mechanisms allow for precise control and adaptation of cellular signaling.
8. Dysregulated Protein Phosphorylation in Disease
Dysregulated protein phosphorylation is a common feature in many diseases, contributing to pathological changes in cellular signaling, metabolism, and function.
8.1 Cancer
Dysregulated protein phosphorylation is a hallmark of cancer. Aberrant activation or inactivation of kinases and phosphatases, as well as mutations in phosphorylation sites or regulatory proteins, can lead to uncontrolled cell proliferation, evasion of apoptosis, and metastasis [10]. Targeting specific phosphorylation events or signaling pathways has emerged as a promising therapeutic strategy in cancer treatment.
8.2 Neurodegenerative Disorders
Protein phosphorylation abnormalities have been implicated in neurodegenerative disorders such as Alzheimer's disease, Parkinson's disease, and Huntington's disease. Dysregulation of kinase activity, altered phosphorylation of tau protein, and impaired signaling pathways contribute to neuronal dysfunction and cell death in these disorders.
8.3 Metabolic Disorders
Disruptions in protein phosphorylation contribute to metabolic disorders, including diabetes and obesity. Phosphorylation events regulate insulin signaling, glucose metabolism, lipid homeostasis, and energy balance. Dysregulation of these processes can lead to insulin resistance, impaired glucose uptake, and altered lipid metabolism.
9. Targeting Protein Phosphorylation for Therapeutic Intervention
Targeting protein phosphorylation has emerged as a promising strategy for therapeutic intervention in various diseases. Modulating cellular signaling pathways and restore normal cellular function by manipulating the activity of protein kinases and phosphatases.
9.1 Kinase Inhibitors
Pharmaceutical research has focused on developing kinase inhibitors as targeted therapeutics. Small molecule inhibitors that selectively target specific kinases have shown success in treating certain types of cancer and other diseases characterized by dysregulated phosphorylation. However, challenges such as target selectivity, drug resistance, and off-target effects remain to be addressed.
9.2 Phosphatase Modulation
Modulating the activity of phosphatases is another potential avenue for therapeutic intervention. However, the development of phosphatase-specific modulators poses significant challenges due to the highly conserved nature of phosphatase catalytic domains.
9.3 Challenges and Future Perspectives
While targeting protein phosphorylation pathways holds promise for therapeutic intervention, several challenges need to be addressed. These include understanding the complexity of signaling networks, improving target selectivity and efficacy, overcoming drug resistance, and developing strategies for specific delivery and tissue targeting. Advancements in technologies such as proteomics, high-throughput screening, and computational modeling will continue to enhance our understanding of protein phosphorylation and pave the way for novel therapeutic approaches.
In summary, protein phosphorylation is a dynamic and highly regulated process that plays a central role in cellular signaling and regulation. It affects protein structure, function, and protein-protein interactions, thus controlling diverse biological processes such as signal transduction, cell cycle regulation, gene expression, and cellular responses.
Advancements in our understanding of protein phosphorylation and its intricate network of signaling pathways will continue to unravel the complexity of cellular biology and disease mechanisms. Further research and technological advancements are needed to decipher the complete phosphoproteome, identify new targets, and develop innovative therapeutic strategies. Harnessing the power of protein phosphorylation holds immense potential for advancing our understanding of human health and disease, ultimately leading to improved diagnostics and targeted therapies.
References
[1] Ubersax JA, Ferrell JE. Mechanisms of specificity in protein phosphorylation [J]. Nature Reviews Molecular Cell Biology. 2007;8(7):530–541.
[2] Eli S Groban, Arjun Narayanan, and Matthew P Jacobson. Conformational Changes in Protein Loops and Helic [J]. PLoS Comput Biol. 2006 Apr; 2(4): e32.
[3] Pincus D, Pandey JP, et al. Evolution and Engineering allosteric regulation in protein kinases [J]. Sci Signal. 2018 Nov 6;11(555):eaar3250.
[4] Alberts B, Johnson A, et al. Molecular Biology of the Cell. Anderson M and Granum S: 5th edition [J]. Garland Science; New York, NY: pp. 1752007
[5] Roskoski R Jr. ERK1/2 MAP kinases: structure, function, and regulation [J]. Pharmacol Res. 66:105–143. 2012.
[6] McCance KL and Huether SE. Pathophysiology: The Biologic Basis for Disease in Adults and Children [J]. Brashers VL and Rote NS: 7th edition. Elsevier; 2014.
[7] Barford D. Molecular mechanisms of the protein serine/thre-onine phosphatases [J]. Trends Biochem Sci. 21:407–412. 1996.
[8] Zhang ZY. Protein tyrosine phosphatases: structure and function, substrate specificity, and inhibitor development [J]. Annu Rev Pharmacol Toxicol. 42:209–234. 2002.
[9] Chang L and Karin M. Mammalian MAP kinase signalling cascades [J]. Nature. 410:37–40. 2001.
[10] Harsha HC and Pandey A. Phosphoproteomics in cancer [J]. Mol Oncol. 4:482–495. 2010.
CUSABIO team. Protein Phosphorylation: The Dynamic Language of Cellular Regulation. https://www.cusabio.com/c-21122.html




Comments
Leave a Comment