T cells, the guardians of our immune system, play a pivotal role in defending our body against infections and diseases. T cells need to be activated to perform their crucial role in the immune response. The intricate activation mechanism not only ensures that our immune response is targeted and robust but also provides long-lasting protection through the formation of memory cells.
Understanding the activation mechanism of T cells can provide in-depth insights into the working principles of the immune system, optimize immunotherapies to improve efficacy and reduce side effects, and guide the design of vaccines to effectively stimulate the immune system and provide lasting protection.
Table of Contents
1. What Are T Cells?
T cells, or T lymphocytes, are a type of white blood cells in the adaptive immune system. They are responsible for identifying and eliminating various pathogens such as bacteria and viruses, as well as for detecting and destroying cancerous or infected cells.
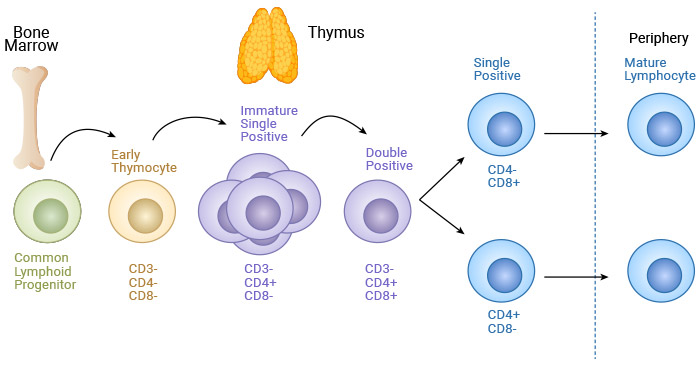
T cells originate from common lymphoid progenitors (CLPs) that differentiate from bone marrow hematopoietic stem cells (HSCs) [1]. CLPs migrate to the thymus, where they undergo differentiation, selection, maturation, and subsequent export to the peripheral tissues [1,2]. These released cells are naïve T cells and must be activated to begin effector functions.
Figure 1. T cell maturation
This picture is cited from: https://www.mdpi.com/2076-2607/9/6/1177
2. Major Types of T Cells and Their Functions
Naïve T cells are activated and differentiated into two broad types: CD8+ T cells and CD4+ T cells. CD8+ T cells, also called cytotoxic T cells or killer T cells, directly kill or destroy infected or cancerous cells. CD4+ T cells, also known as helper T (Th) cells, do not directly kill cells rather than sending signals that direct other immune cells to fight against invading pathogens.
| Classification |
Function |
| Cytotoxic T cells |
Induce apoptosis of the target cell through the Fas-FasL pathway;
Release perforin to form pores in the target cell membrane and granzymes to induce apoptosis;
Release IFNγ and TNF-α, which are important in defending against viral infections and in controlling the proliferation of tumoral cells [15] |
| Helper T cells |
Th1 |
Important in controlling intracellular bacterial and viral infections;
Produce large and persistent amounts of IFN-γ and and TNF-α, which activate macrophages to phagocytose intercellular bacteria |
| Th2 |
Mainly involved in helminth infections;
Produce cytokines like IL-4, IL-5, and IL-13, which activate neighboring eosinophils, mast cells, and basophils to clear the parasitic infection [3,4] |
| Th9 |
Promote IgE class switching, mast cell recruitment, and mucus generation |
| Th17 |
Important in mucosal bacterial and fungal responses;
Secrete IL17A, IL17F, and IL-22, which recruits neutrophils and monocytes to sites of infection [12] |
| T Regulatory cell (Treg cells or suppressor T cells) |
Generate IL-10 and IL-35 to inhibit overactive immune responses;
Essential for maintaining peripheral tolerance, preventing autoimmunity and limiting chronic inflammatory diseases |
| Folicular helper (Tfh) |
Express various cytokines and co-stimulatory molecules to aid in the germinal center reaction of B cells, facilitating the production of high-affinity antibodies [13,14] |
T cells express the T cell receptor (TCR) on their surface during the maturation process. The TCR is a transmembrane heterodimer and exists in two types: TCRαβ and TCRγδ, with a single antigen-binding site. An individual T cell bears either an αβ or a γδ heterodimer as its receptor, but never both. The TCR is always expressed with CD3 complex, which is dispensable for signal transduction upon recognition of the presented antigen [9].
The TCRαβ chains are found on the majority of peripheral T cells, which are called αβ T cells, and the others are termed γδ T cells. The TCRαβ recognizes processed liner peptides bound to the major histocompatibility complex (MHC), whereas TCRγδ recognizes unprocessed antigens.
3. What Is T Cell Activation?
T cell activation is a complex process that transforms a naïve T cell into a formidable fighter T cell. This process involves multiple steps and signals that ensure the precise activation of T cells in response to specific pathogens.
3.1 What Initiates T Cell Activation?
T cells only respond to cells presenting both a self-MHC molecule and a foreign antigenic determinant. T cell activation is initiated by the action of two signals and cytokines: i) antigen stimulation signal, ii) co-stimulatory signal, and iii) involvement of cytokines that initiate clonal expansion [5].
3.2 How Are T Cells Activated?
T cell activation involves antigen-presenting and recognition, co-stimulation, signal transduction, clonal expansion and differentiation into effector T cells, migration to infected sites, performing effector functions, and formation of memory T cells.
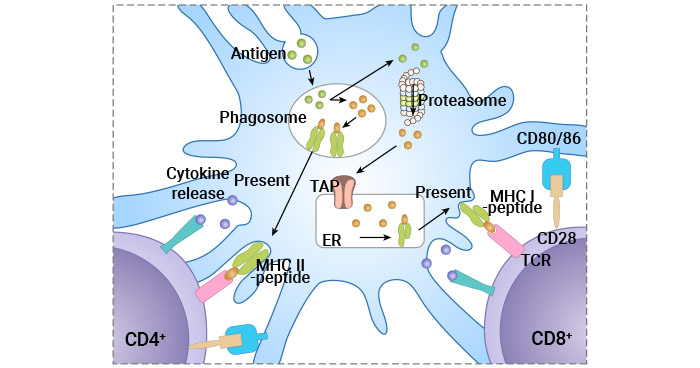
Figure 2. Antigen-specific T cell activation
This picture is cited from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9316852/
① Antigen Presenting
Antigen presentation includes antigen uptake, antigen processing, and MHC molecule loading. Antigen-presenting cells (APCs) such as dendritic cells (DCs), macrophages, and B cells play a crucial role in T cell activation.
APCs capture antigens from pathogens or pathogen-derived proteins through phagocytosis or endocytosis and then process them into small peptide fragments. These antigenic fragments noncovalently bind to MHC molecules to form the peptide-MHC (pMHC) complexes in the rough endoplasmatic reticulum and are transported to the cell surface for presentation [10].
② Antigen Recognition
The TCR on the surface of T cells recognizes and binds to the peptide-MHC complex on APCs. CD4+ T cells recognize antigens such as bacterial peptides presented by MHC class II molecules. CD8+ T cells recognize antigens such as viral peptides presented by MHC class I molecules.
However, the TCR lacks intracellular signaling domains. It associates with CD3 ( composed of γ-, δ-, ε-, and ζ-subunits), which transmits the TCR-triggered signal through immunoreceptor tyrosine-based activation motifs (ITAMs) in its cytosolic region [11].
③ Co-Stimulatory Signals
The engagement of TCR with the peptide-MHC complex provides the first signal for T cell activation. A second co-stimulatory signal is necessary to fully activate the T cells and prevent anergy or apoptosis.
The CD28 on T Cells binds to B7 molecules (CD80 and CD86) on APCs, providing crucial co-stimulatory signals. The activation signal of these costimulatory molecules is conveyed to T cells via the ITAM of the cytoplasmic domain, boosting the TCR response to antigen.
The absence of co-stimulation leads to T cell anergy or apoptosis, ensuring that T cells are only activated in the presence of infection or danger signals. Lack of co-stimulatory signals is one of the important reasons for tumor cells to evade the surveillance of the body's immune system.
④ Signal Transduction Pathways
Upon receiving both the primary and co-stimulatory signals, a cascade of intracellular signaling pathways is activated. The T cell receptor signaling pathway leads to the transcription of genes necessary for T cell proliferation, differentiation, and effector functions.
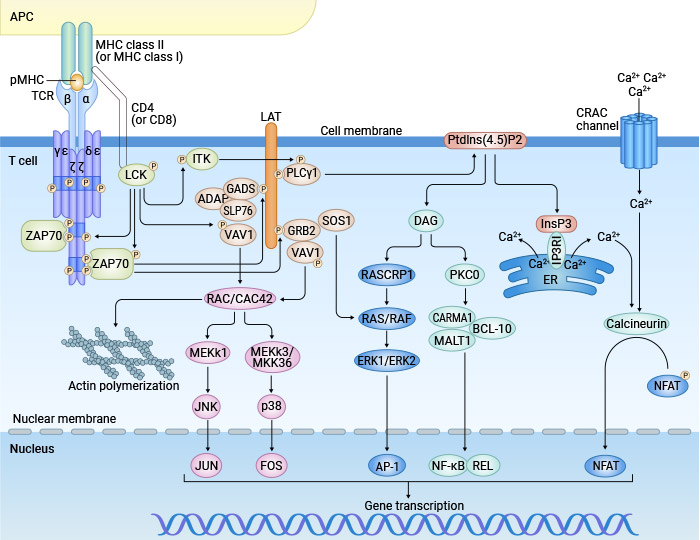
Calcium Signaling: PLCγ1 converts the PIP2 into IP3 and diacylglycerol (DAG). The IP3 diffuses into the cytoplasm and ligates to the receptors of the endoplasmic reticulum, where it induces the release of Ca2+ deposits to the cytosol. The intracellular increase of Ca2+ activates calcineurin, which dephosphorylates NFAT, allowing it to translocate to the nucleus and promote the transcription of IL-2 and other cytokines [6].
MAPK pathway: ZAP-70 phosphorylates and activates LAT, which recruits several proteins that facilitate the transference of guanine nucleotides from GDP to GTP for the activation of Ras, thus initiating a cascade of phosphorylations. These events lead to the activation of MAPK, which activates the transcription factor AP-1 composed of the proteins Jun, Fos, and activating transcription factor (ATF). MAPKs allow dimerization of those proteins to initiate the transcription of genes required for T cell proliferation and differentiation [7].
NF-κB Pathway: The generation of DAG activates protein kinase C (PKC), which stimulates the activation of the NF-κB pathway, resulting in the transcription of genes involved in T cell survival and proliferation [8].
Figure 3. Signaling pathways involved in T cell activation
This picture is cited from: https://pubmed.ncbi.nlm.nih.gov/29789755/
⑤ Clonal Expansion and Differentiationinto Effector T cells
Activated naïve T cells undergo rapid proliferation and differentiation into various subsets with distinct functions. This clonal expansion ensures that a sufficient number of T cells are available to effectively combat the pathogen.
CD4+T cellsdifferentiate into several subsets, including Th1, Th2, Th17, and Treg cells, each producing different cytokines and performing various roles in the immune response.CD8+T cellsdifferentiate into cytotoxic T lymphocytes (CTLs) that can directly kill infected or cancerous cells by releasing perforin and granzymes.
⑥ Migration to Infected Sites
Activated T cells, including CD4+and CD8+subsets, travel through the bloodstream to the site of infection or inflammation. Their migration to specific tissues is guided by chemokines and adhesion molecules.
⑦ Effector Functions of Activated T Cells
Activated T cells carry out their effector functions, eliminatingthe pathogen and infected cells. These functions vary between CD4+and CD8+T cells.
CD4+T Cell Effector Functions:
Cytokine Secretion: Help activate and regulate other immune cells. For instance, IFN-γ produced by Th1 cells activates macrophages to enhance their microbicidal activity.
B Cell Activation: Th2 cells produce IL-4, IL-5, and IL-13, which helpsB cells undergo class switching and affinity maturation to generatehigh-affinity antibodies.
Inflammation Promotion: Th17 cells produce IL-17, which recruits neutrophils and promotes inflammation.
Immune Tolerance Maintenance: Treg cells generate TGF-β and IL-10, which is conducive to maintaining immune tolerance.
CD8+T Cell Effector Functions:
Direct Killing: CTLs kill infected cells by inducing apoptosis through the release of perforin and granzymes.
Cytokine Production: CTLs produce cytokines like IFN-γ to enhance the immune response.
⑧ Formation of Memory T Cells
After the clearance of the infection, most effector T cells undergo apoptosis. However, a subset of Tcells differentiates into memory T cells, which provide long-lasting immunity and rapid response upon re-exposure to the same antigen.
Central memory T cells reside in lymphoid tissues and can proliferate extensively upon re-exposure to the antigen.Effector memory T cells circulate in peripheral tissues and provide immediate protection upon encountering the antigen again.
4. T Cell Activation Markers
T cell markers are specific molecules expressed on the surface or within T cells that indicate their activation status. Common T cell activation markers include:
| Marker |
Function |
Detection Method |
| CD69 |
An early activation marker rapidly upregulated on the surface of T cells following activation. It indicates the initiation of the activation process. |
Flow cytometry |
| CD25 (IL-2 Receptor Alpha) |
Upregulated after T cell activation, indicating the T cell's readiness to respond to IL-2 for proliferation. |
Flow cytometry |
| CD44 |
A cell adhesion molecule that is upregulated on activated T cells, involved in cell migration and homing to sites of inflammation. |
Flow cytometry |
| CD40L (CD40 Ligand, also known as CD154) |
Expressed on activated CD4+ T cells, involved in the activation of B cells, macrophages, and dendritic cells. |
Flow cytometry |
| CTLA-4 |
An inhibitory receptor that is upregulated on activated T cells, providing a negative feedback mechanism to regulate T cell activation. |
Flow cytometry |
| PD-1 |
An inhibitory receptor that regulates T cell activation and maintains tolerance, upregulated upon T cell activation. |
Flow cytometry |
| IFN-γ (IFNG) |
A cytokine produced by activated T cells, especially Th1 and CTLs, involved in activating macrophages and promoting the immune response. |
Intracellular cytokine staining followed by flow cytometry, ELISA of culture supernatants |
| IL-2 |
A cytokine critical for T cell proliferation and survival, produced by activated T cells. |
Intracellular cytokine staining followed by flow cytometry, ELISA of culture supernatants |
| TNF-α |
A cytokine produced by activated T cells that promotes inflammation and apoptosis in target cells. |
Intracellular cytokine staining followed by flow cytometry, ELISA of culture supernatants |
| Granzyme B (GZMB) |
A serine protease produced by activated CD8+ T cells (CTLs) involved in inducing apoptosis in target cells. |
Intracellular staining followed by flow cytometry |
| Ki-67 |
A nuclear protein associated with cell proliferation, indicating that T cells are actively dividing. |
Intracellular staining followed by flow cytometry |
| Phosphorylated ZAP-70 |
Indicates activation of the TCR signaling pathway, as ZAP-70 is phosphorylated upon TCR engagement. |
Intracellular staining followed by flow cytometry, Western blotting |
| Phosphorylated ERK |
Part of the MAPK signaling pathway, indicating activation of downstream signaling following TCR engagement. |
Intracellular staining followed by flow cytometry, Western blotting |
These methods offer various advantages and disadvantages, as well as suitability for the purification of different types of antibody.
5. Methods for T Cell Activation in Vitro
T cell activation is a critical step in the immune response and the cornerstone of the development of T cell-related therapies. As CD3 and CD28 are two important targets for T cell activation, the combined use of CD3 antibody and CD28 antibody to stimulate T cells in vitro can simulate the double-signaling effect of T cell activation in vivo.
Several methods stimulate and induce T cell activation in vitro by using CD3 and CD28 antibodies, including direct use of functional antibodies, antibody-coupled magnetic beads, and antibody-conjugated Streptamer polymers, which are conducive to understanding how T cells respond to antigens and developing therapeutic interventions.
5.1 T cell isolation and cultivation
T cells can be isolated from peripheral blood, spleen, or lymph nodes of an animal (such as a mouse) using various techniques such as density gradient centrifugation or magnetic-activated cell sorting (MACS). The isolated T cells are cultivated in the cell culture medium with IL-2 to promote their proliferation.
5.2 T cell stimulation
Add CD3 and CD28 antibodies (CD3-CD28 antibody-coupled magnetic beads or CD3/CD28 antibody-conjugated Streptamer polymers) to the cell culture medium to simulate two activation signals. Alternatively, commercial activation amplification kits can be used to stimulate the activation of T cells. If needed, cytokines such as IL-2 can be added simultaneously to enhance T cell activation and proliferation.
Cell status should be observed regularly during culture and new media or cytokines should be added as needed. The stimulation reaction is terminated when activators (antibodies, magnetic beads, or polymers) are removed from the activated T cells.
5.3 Activation marker detection
The activation status of T cells is evaluated by flow cytometry for surface activation markers such as CD25 and CD69 or by ELISA for cytokines in culture supernatants such as IFN-γ and IL-2. Increased expression of CD69 and CD25 on CD8+ T cells indicates successful activation. Elevated levels of IFN-γ and IL-2 in the supernatants confirm cytokine production.
5.4 Cell proliferation detection
Detect T cell proliferation by cell counting, MTT method, CFSE staining, and other methods. Significant dilution of CFSE dye demonstrates robust proliferation of CD8+ T cells.
6. T Cell Activation and Immunotherapy
T cell activation is at the heart of many immunotherapy strategies, which leverage the body's immune system to fight diseases, especially cancer. By understanding and manipulating the pathways that control T cell activation, researchers and clinicians can develop more effective cancer therapies.
6.1 Chimeric Antigen Receptor (CAR) T-Cell Therapy
CAR T-cell therapy involves genetically engineering a patient's T-cells to express a fully synthetic chimeric antigen receptor (CAR) that targets a specific antigen on cancer cells [16]. These modified T-cells are expanded in the lab and infused back into the patient, where they can be activated by the specific antigen on the cancer cells and then target and eliminate these cancer cells more effectively. This therapy is highly reliant on robust T-cell activation for its effectiveness.
T cell activation is tightly regulated by immune checkpoints, such as CTLA-4 and PD-1, which serve as brakes to prevent overactivation of the immune system. Tumor cells evade immune attack by expressing PD-L1 or other immune checkpoint molecules to suppress T cell activation. In immune checkpoint therapies, immune checkpoint inhibitors are used to block these inhibitory pathways, thereby enhancing T-cell activation and enabling the immune system to target and destroy cancer cells more effectively [17].
6.3 Tumor infiltrating lymphocytes (TIL) therapy
In some cancers, T cells can infiltrate tumor tissue naturally, but their numbers or activity may not be sufficient to completely remove cancer cells. In TIL therapy, tumor-specific T cells are extracted from a patient, activated and expanded in the laboratory, and then reinfused into the patient to enhance the immune response [18]. This method aims to boost the number of activated T-cells that can fight cancer cells.
6.4 Cancer Vaccines
Cancer vaccines are designed to prevent or treat cancer by inducing or enhancing the immune response of T cells to tumor antigens. These vaccines typically contain cancer-specific antigens (neoantigens [neoAg]) that are used to prime T cells, promoting their activation, migration, and infiltration into tumors, as well as recognition and killing of cancer cells [19].
Conclusion
The activation of T cells is a highly regulated process that ensures the immune system can effectively respond to pathogens while avoiding unnecessary or harmful responses. Understanding the detailed mechanisms of T cell activation is crucial for developing targeted immunotherapies and vaccines that can enhance or modulate the immune response to various diseases.
References
[1] Ghaedi, M. et al. Common-lymphoid-progenitor-independent pathways of innate and T lymphocyte development [J]. Cell Rep. 15, 471–480 (2016).
[2] Starr, T. K., Jameson, S. C. & Hogquist, K. A. Positive and negative selection of T cells [J]. Annu. Rev. Immunol. 21, 139–176 (2003).
[3] Williams JW, Ferreira CM, et al. Non-apoptotic Fas (CD95) Signaling on T Cells Regulates the Resolution of Th2-Mediated Inflammation [J]. Front Immunol. 2018;9:2521.
[4] Hirata H, Yukawa T, et al. Th2 cell differentiation from naive CD4+ T cells is enhanced by autocrine CC chemokines in atopic diseases [J]. Clin Exp Allergy. 2019 Apr;49(4):474-483.
[5] Kapsenberg ML. Dendritic-cell control of pathogen-driven T Cell polarization [J]. Nat Rev Immunol. 2003;3:984–93.
[6] Muller MR, Rao A. NFAT, immunity and cancer: a transcription factor comes of age [J]. Nat Rev Immunol. 2010;10:645–56.
[7] Brownlie RJ, Zamoyska R. T cell receptor signalling networks: branched, diversified and bounded [J]. Nat Rev Immunol. 2013;13:257–69.
[8] Siebenlist U, Brown K, Claudio E. Control of lymphocyte development by nuclear factor-kappaB [J]. Nat Rev Immunol. 2005;5:435–45.
[9] Call ME, Wucherpfennig KW 2005. The T cell receptor: Critical role of the membrane environment in receptor assembly and function [J]. Annu Rev Immunol 23:101–125.
[10] Neefjes, J., Jongsma, et al. (2011). Towards a systems understanding of MHC class I and MHC class II antigen presentation [J]. Nat. Rev. Immunol. 11, 823–836.
[11] Letourneur, F. & Klausner, R. D. Activation of T cells by a tyrosine kinase activation domain in the cytoplasmic tail of CD3 epsilon [J]. Science 255, 79–82 (1992).
[12] Mangan PR, Harrington LE, et al. Transforming growth factor-β induces development of the TH17 lineage [J]. Nature 441: 231–234, 2006.
[13] Breitfeld D, Ohl L, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production [J]. J Exp Med 192: 1545–1552, 2000.
[14] Crotty S. Follicular helper CD4 T cells (TFH) [J]. Annu Rev Immunol 29: 621–663, 2011.
[15] Cox MA, Kahan SM, Zajac AJ. Anti-viral CD8 T cells and the cytokines that they love [J]. Virology. 2013;435:157–69.
[16] Uscanga-Palomeque AC, Chávez-Escamilla AK, et al. CAR-T Cell Therapy: From the Shop to Cancer Therapy [J]. Int J Mol Sci. 2023 Oct 28;24(21):15688.
[17] Wang, M.M., Coupland, S.E., et al. Resistance to immune checkpoint therapies by tumour-induced T-cell desertification and exclusion: key mechanisms, prognostication and new therapeutic opportunities [J]. Br J Cancer 129, 1212–1224 (2023).
[18] Zhao Y, Deng J, et al. Tumor Infiltrating Lymphocyte (TIL) Therapy for Solid Tumor Treatment: Progressions and Challenges [J]. Cancers (Basel). 2022 Aug 27;14(17):4160.
[19] Fan, T., Zhang, M., Yang, J. et al. Therapeutic cancer vaccines: advancements, challenges, and prospects [J]. Sig Transduct Target Ther 8, 450 (2023).
CUSABIO team. T Cell Activation - The Switch for T Cell Executive Function. https://www.cusabio.com/c-21187.html




Comments
Leave a Comment