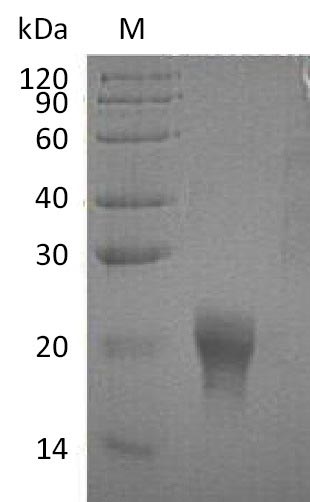
Recombinant Human Interleukin-5 (IL5) is expressed in mammalian cells and contains the complete mature protein sequence from amino acids 20 to 134. A 6xHis-tag has been added to the C-terminus to simplify purification and detection processes. SDS-PAGE analysis indicates purity levels above 95%. Endotoxin levels stay below 1.0 EU/µg, which appears to minimize potential experimental interference. The biological activity shows an ED50 of 0.1-0.5 ng/ml when tested in TF-1 cell proliferation assays.
Interleukin-5 (IL5) serves as a cytokine that mainly controls eosinophil regulation and activation, contributing significantly to immune responses. It acts as an essential element in signaling pathways connected to allergic inflammation. Scientists frequently examine IL5 when studying asthma and other conditions involving eosinophils. Research into IL5's mechanisms may offer valuable insights for developing therapeutic approaches that can modify immune responses.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
1. Cell Proliferation and Viability Assays for Eosinophil Research
This recombinant IL-5 is confirmed to be highly bioactive (ED₅₀ 0.1-0.5 ng/ml in TF-1 cells) and suitable for eosinophil studies. However, researchers should validate its activity in primary human eosinophils to confirm cross-reactivity, as TF-1 cells are erythroleukemic and may have different receptor expression/ signaling compared to primary eosinophils. The mammalian expression ensures proper glycosylation, critical for IL-5's stability and in vivo half-life, supporting physiological relevance. The C-terminal His-tag is unlikely to significantly impact proliferation readouts
2. IL-5 Receptor Binding and Interaction Studies
The protein is appropriate for receptor-binding studies, but the C-terminal His-tag may cause minor steric effects near the receptor-binding interface, potentially altering binding kinetics. While mammalian folding supports authentic interactions, researchers should validate affinity constants (Kd) with tag-free IL-5 for precise measurements. The high potency confirms proper folding of the receptor-binding domains.
3. Antibody Development and Characterization
This mammalian-expressed IL-5 serves as an excellent antigen due to proper folding and glycosylation. However, the His-tag may induce tag-specific antibodies, so screening should include tag-free controls. Antibodies should be validated for their ability to neutralize IL-5 bioactivity in primary eosinophil assays, not just TF-1 cells, to ensure physiological relevance.
4. Signal Transduction Pathway Analysis
The biologically active IL-5 is suitable for signaling studies (e.g., JAK-STAT, particularly STAT5 activation). The mammalian glycosylation may influence receptor complex stability and signal duration. Researchers should confirm that phosphorylation kinetics in primary eosinophils match those in TF-1 cells, as signaling dynamics may differ between cell types.
5. Competitive Inhibition and Drug Screening Assays
The protein is ideal for high-throughput screening, but the C-terminal tag might affect the binding of some small-molecule inhibitors if they interact with the C-terminal region. Hit compounds should be validated against tag-free IL-5. The low ED₅₀ allows for low-concentration screening, reducing compound and protein consumption.
Final Recommendation & Action Plan
This mammalian-expressed, C-terminal His-tagged human IL-5 is a high-quality reagent with confirmed high potency, making it suitable for all proposed applications. However, the His-tag necessitates validation for binding and inhibition studies. For immediate use, employ it at low concentrations (0.1-1.0 ng/ml) based on the ED₅₀. For eosinophil research, first establish a dose-response in primary cells, as potency may differ from TF-1 cells. For binding studies, the tag facilitates immobilization but may slightly alter kinetics; consider including tag-free IL-5 as a control if precise affinity measurement is required. When developing antibodies, this protein is ideal due to its native-like glycosylation, but use a tag-cleaved protein for immunization to avoid tag-specific antibodies. For signaling studies, the mammalian expression ensures physiological glycosylation, which is important for proper receptor dimerization and signaling amplitude. For drug screening, the protein is excellent for initial high-throughput assays, but prioritize validation of hits with tag-free IL-5. The low endotoxin level is crucial for cell-based assays to avoid non-specific activation. Always include relevant controls (e.g., unstimulated cells, tag-only controls) to ensure specificity.