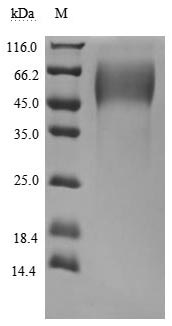
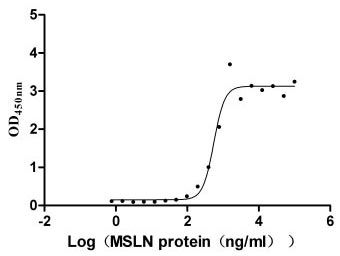
This biologically active recombinant human Mucin-16 (MUC16) protein contains a specific fragment (amino acids 12660-12923) of the human MUC16, produced in mammalian cells with C-terminal 6×His tagging. The recombinant MUC16 protein demonstrates exceptional purity (>95% by SDS-PAGE) and meets stringent endotoxin specifications (<1.0 EU/μg, LAL method), ensuring research-grade quality for sensitive applications. Functional characterization reveals its specific binding capacity to MSLN (CSB-MP015044HUc9) in ELISA formats, with an EC50 range of 460.7-662.2 ng/ml when immobilized at 10 μg/ml. The mammalian expression system ensures proper post-translational modifications critical for maintaining MUC16's native structural characteristics. Presented as lyophilized powder, this preparation offers enhanced stability and convenient reconstitution properties. The C-terminal 6×His tag facilitates straightforward purification and detection procedures while preserving protein functionality. This recombinant MUC16 protein is an essential tool for investigating mucin-mediated cellular interactions, particularly in studies of cancer biomarkers, tumor microenvironment modulation, and the development of diagnostic or therapeutic approaches targeting MUC16-associated pathways.
Human MUC16, also known as the ovarian cancer antigen CA125, is a member of the mucin family of glycoproteins, characterized by its large molecular weight ranging from 3 to 5 million Daltons [1][2]. MUC16 is predominantly expressed on the surface of epithelial cells, particularly in the ovaries, and plays multiple roles in normal physiology and disease processes, including cancer [3]. The MUC16 protein consists of three primary regions: a large N-terminal domain, a central domain with numerous glycosylated tandem repeats, and a C-terminal domain that anchors it to the cell membrane [2][3].
Functionally, MUC16 is involved in cell signaling and contributes to tumor biology in several malignancies, particularly ovarian cancer. It serves as a biomarker for ovarian cancer, where high serum levels of CA125 can be indicative of disease presence or progression [4][5]. Moreover, MUC16 interacts with other proteins, including mesothelin, which further promotes cancer cell adhesion to mesothelial surfaces and enhances metastatic potential [3][6]. Evidence suggests that MUC16 may also modulate immune responses by engaging receptors on immune cells, which can lead to immune evasion by cancer cells [5][7].
Additionally, MUC16's structural attributes include substantial glycosylation, which not only affects its interaction profiles but also may impact immune recognition and response [8]. Alterations in MUC16 expression and glycosylation patterns can correlate with more aggressive cancer phenotypes and poorer prognoses [9]. Recent studies have further elucidated the roles of MUC16 in cell transformation and invasion, indicating that it may promote epithelial-to-mesenchymal transition (EMT) processes [10].
References:
[1] A. Shimizu, S. Hirono, et al. Coexpression of muc16 and mesothelin is related to the invasion process in pancreatic ductal adenocarcinoma. Cancer Science, vol. 103, no. 4, p. 739-746, 2012. https://doi.org/10.1111/j.1349-7006.2012.02214.x
[2] B. White, M. Patterson, S. Karnwal, & C. Brooks. Crystal structure of a human muc16 sea domain reveals insight into the nature of the ca125 tumor marker. Proteins Structure Function and Bioinformatics, vol. 90, no. 5, p. 1210-1218, 2022. https://doi.org/10.1002/prot.26303
[3] S. Das and S. Batra. Understanding the unique attributes of muc16 (ca125): potential implications in targeted therapy. Cancer Research, vol. 75, no. 22, p. 4669-4674, 2015. https://doi.org/10.1158/0008-5472.can-15-1050
[4] T. Imamura, Y. Okamura, et al. Overview and clinical significance of multiple mutations in individual genes in hepatocellular carcinoma. BMC Cancer, vol. 22, no. 1, 2022. https://doi.org/10.1186/s12885-022-10143-z
[5] Y. Huang, X. Huang, J. Zeng, & J. Lin. Knockdown of muc16 (ca125) enhances the migration and invasion of hepatocellular carcinoma cells. Frontiers in Oncology, vol. 11, 2021. https://doi.org/10.3389/fonc.2021.667669
[6] S. Chen, M. Dallas, E. Balzer, & Κ. Κωνσταντόπουλος. Mucin 16 is a functional selectin ligand on pancreatic cancer cells. The Faseb Journal, vol. 26, no. 3, p. 1349-1359, 2011. https://doi.org/10.1096/fj.11-195669
[7] J. Belisle, S. Horibata, et al. Identification of siglec-9 as the receptor for muc16 on human nk cells, b cells, and monocytes. Molecular Cancer, vol. 9, no. 1, 2010. https://doi.org/10.1186/1476-4598-9-118
[8] C. Wang, E. Hanson, L. Minkoff, & R. Whelan. Individual recombinant repeats of muc16 display variable binding to ca125 antibodies. Cancer Biomarkers, vol. 37, no. 2, p. 85-94, 2023. https://doi.org/10.3233/cbm-220191
[9] X. Yang, S. Zhu, et al. Identification of differentially expressed genes and signaling pathways in ovarian cancer by integrated bioinformatics analysis. Oncotargets and Therapy, vol. Volume 11, p. 1457-1474, 2018. https://doi.org/10.2147/ott.s152238
[10] M. Comamala, M. Pinard, et al. Downregulation of cell surface ca125/muc16 induces epithelial-to-mesenchymal transition and restores egfr signalling in nih:ovcar3 ovarian carcinoma cells. British Journal of Cancer, vol. 104, no. 6, p. 989-999, 2011. https://doi.org/10.1038/bjc.2011.34