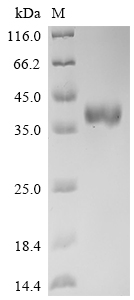
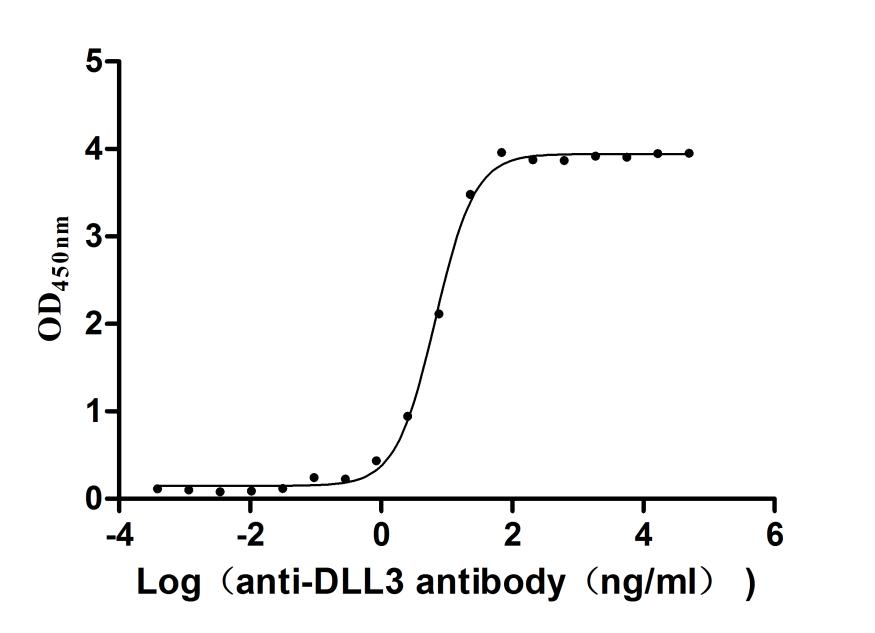
The recombinant human DLL3 protein is produced by transfecting mammalian cells with a plasmid containing the gene segment encoding the 429-492aa region of the human DLL3. The target gene segment is co-expressed with the C-terminal mFc-tag gene. SDS-PAGE analysis verifies a purity level of this DLL3 protein above 95%. The functional activity of this recombinant DLL3 protein is validated through ELISA, with specific DLL3 recombinant antibody (CSB-RA882142MA2HU) binding showing an EC50 range of 6.211 to 7.209 ng/mL.
Human DLL3 is a member of the Delta/Serrate/LAG-2 (DSL) family of proteins, which play critical roles in the Notch signaling pathway. Unlike other DSL ligands, DLL3 does not activate Notch signaling but instead acts as a negative regulator, inhibiting the signaling induced by other DSL ligands such as DLL1 [1][2]. This unique function positions DLL3 as a significant player in various biological processes, particularly in neurogenesis and the development of certain cancers.
DLL3 is predominantly expressed in neuroendocrine tumors, especially small cell lung cancer (SCLC), where it serves as a potential biomarker and therapeutic target. Studies have shown that DLL3 is highly expressed in SCLC, with approximately 72% of treatment-naïve cases exhibiting positive DLL3 expression [3][4]. Furthermore, DLL3 expression is associated with poor prognosis in various cancers, including endometrial cancer and small cell bladder cancer, where high levels of DLL3 correlate with shorter progression-free survival (PFS) and overall survival (OS) [5][6].
In addition to its role in cancer, DLL3 mutations are implicated in spondylocostal dysostosis (SCD), a genetic disorder characterized by vertebral segmentation defects. Mutations in the DLL3 gene disrupt normal somitogenesis, leading to various skeletal deformities [7-9]. These mutations can result in truncated proteins that fail to function properly, thereby affecting the Notch signaling pathway, which is essential for proper vertebral development [10][11].
References:
[1] E. Ladi, J. Nichols, W. Ge, A. Miyamoto, C. Yao, L. Yang, et al. The divergent dsl ligand dll3 does not activate notch signaling but cell autonomously attenuates signaling induced by other dsl ligands, The Journal of Cell Biology, vol. 170, no. 6, p. 983-992, 2005. https://doi.org/10.1083/jcb.200503113
[2] I. Geffers, K. Serth, G. Chapman, R. Jaekel, K. Schuster-Gossler, R. Cordes, et al. Divergent functions and distinct localization of the notch ligands dll1 and dll3 in vivo, The Journal of Cell Biology, vol. 178, no. 3, p. 465-476, 2007. https://doi.org/10.1083/jcb.200702009
[3] M. Furuta, J. Sakakibara‐Konishi, H. Kikuchi, H. Yokouchi, H. Nishihara, H. Minemura, et al. Analysis of dll3 and ascl1 in surgically resected small cell lung cancer (hot1702), The Oncologist, vol. 24, no. 11, p. e1172-e1179, 2019. https://doi.org/10.1634/theoncologist.2018-0676
[4] D. Morgensztern, B. Besse, L. Greillier, R. Santana-Dávila, N. Ready, C. Hann, et al. Efficacy and safety of rovalpituzumab tesirine in third-line and beyond patients with dll3-expressing, relapsed/refractory small-cell lung cancer: results from the phase ii trinity study, Clinical Cancer Research, vol. 25, no. 23, p. 6958-6966, 2019. https://doi.org/10.1158/1078-0432.ccr-19-1133
[5] J. Wang, K. Zhang, Z. Li, T. Wang, F. Shi, Y. Zhang, et al. Upregulated delta-like protein 3 expression is a diagnostic and prognostic marker in endometrial cancer, Medicine, vol. 97, no. 51, p. e13442, 2018. https://doi.org/10.1097/md.0000000000013442
[6] V. Koshkin, J. García, J. Reynolds, P. Elson, C. Magi‐Galluzzi, J. McKenney, et al. Transcriptomic and protein analysis of small-cell bladder cancer (scbc) identifies prognostic biomarkers and dll3 as a relevant therapeutic target, Clinical Cancer Research, vol. 25, no. 1, p. 210-221, 2019. https://doi.org/10.1158/1078-0432.ccr-18-1278
[7] S. Dunwoodie, M. Clements, D. Sparrow, X. Sa, R. Conlon, & R. Beddington. Axial skeletal defects caused by mutation in the spondylocostal dysplasia/pudgy genedll3are associated with disruption of the segmentation clock within the presomitic mesoderm, Development, vol. 129, no. 7, p. 1795-1806, 2002. https://doi.org/10.1242/dev.129.7.1795
[8] P. Turnpenny, N. Whittock, J. Duncan, S. Dunwoodie, K. Kusumi, & S. Ellard. Novel mutations in dll3, a somitogenesis gene encoding a ligand for the notch signalling pathway, cause a consistent pattern of abnormal vertebral segmentation in spondylocostal dysostosis, Journal of Medical Genetics, vol. 40, no. 5, p. 333-339, 2003. https://doi.org/10.1136/jmg.40.5.333
[9] F. Khan, A. Arshad, A. Ullah, E. Steenackers, G. Mortier, F. Ahmad, et al. Identification of a novel nonsense variant in the dll3 gene underlying spondylocostal dysostosis in a consanguineous pakistani family, Molecular Syndromology, vol. 14, no. 3, p. 191-200, 2023. https://doi.org/10.1159/000527043
[10] L. Bonafé, C. Giunta, M. Gassner, B. Steinmann, & A. Superti‐Furga. A cluster of autosomal recessive spondylocostal dysostosis caused by three newly identified dll3 mutations segregating in a small village, Clinical Genetics, vol. 64, no. 1, p. 28-35, 2003. https://doi.org/10.1034/j.1399-0004.2003.00085.x
[11] N. Whittock, S. Ellard, J. Duncan, C. Die-Smulders, J. Vles, & P. Turnpenny. Pseudodominant inheritance of spondylocostal dysostosis type 1 caused by two familial delta‐like 3 mutations, Clinical Genetics, vol. 66, no. 1, p. 67-72, 2004. https://doi.org/10.1111/j.0009-9163.2004.00272.x