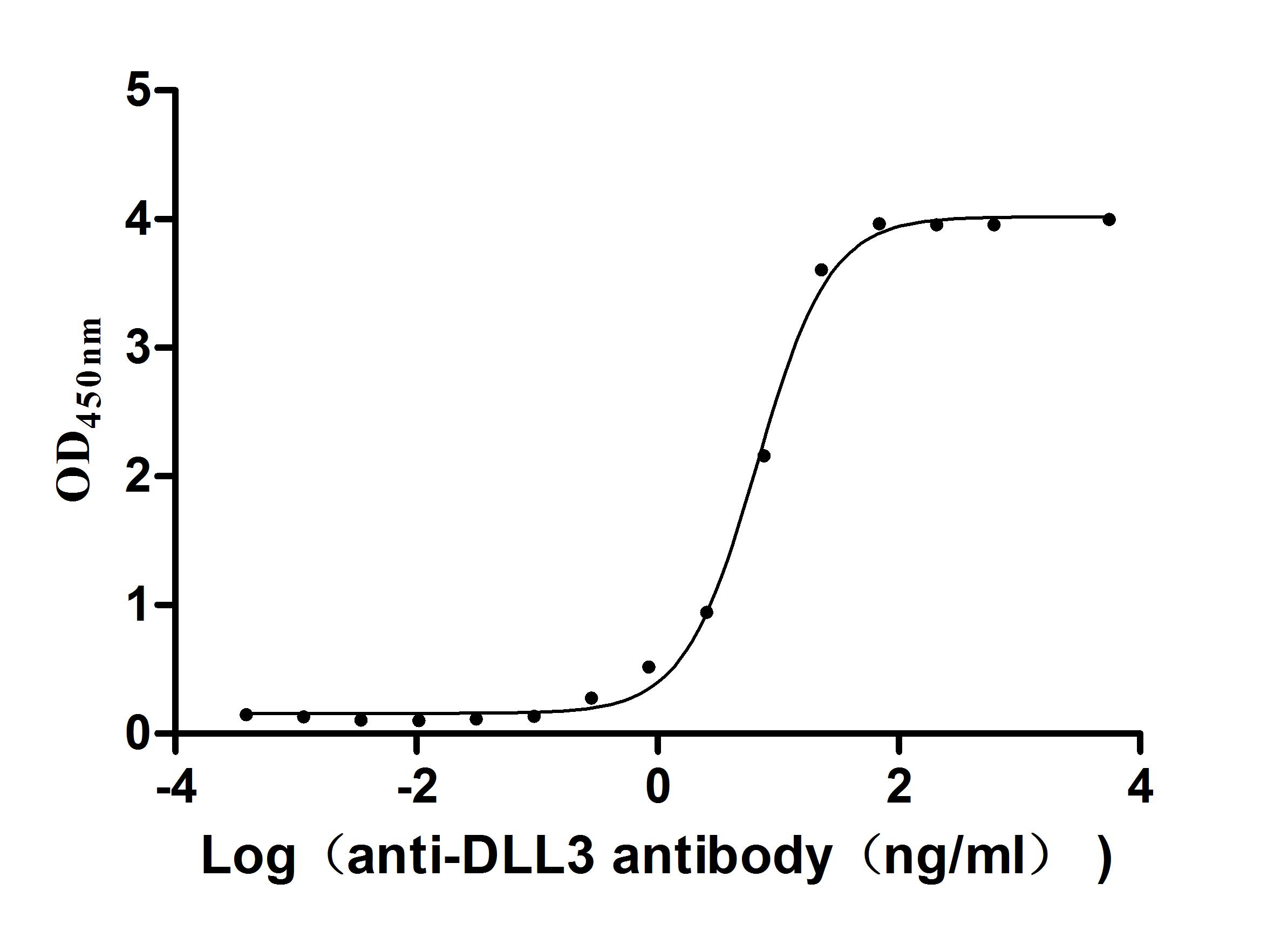
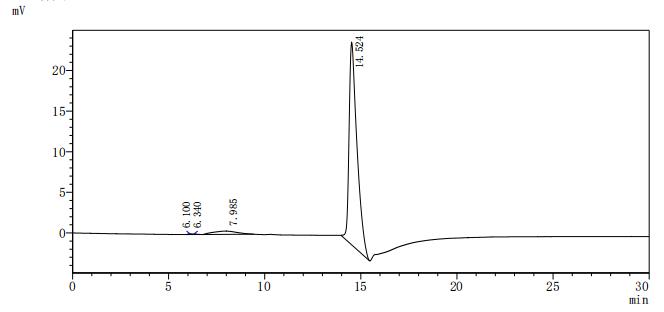
The gene fragment encoding the 391-492aa of the human DLL3 is co-expressed with the C-terminal mFc-tag gene in mammalian cells. The resulting product is the recombinant human DLL3 protein. This DLL3 protein is active as it can bind to the DLL3 recombinant antibody (CSB-RA882142MA2HU), with the EC50 of 6.080-7.240 ng/mL. Its purity is up to 95% as determined by SDS-PAGE.
The human DLL3 protein is a multifunctional protein that regulates Notch signaling, cell proliferation, migration, and invasion, with particular relevance in the context of neuroendocrine tumors. Its unique expression profile and role in tumor biology make it a potential therapeutic target in certain cancer types.
RDLL3 is an atypical Notch ligand that can inhibit Notch receptor activation [1-3]. This inhibition of Notch signaling promotes neuroendocrine differentiation [2]. DLL3 overexpression has been shown to promote tumor growth and inhibit apoptosis in various cancer types, including small cell lung cancer (SCLC) and hepatocellular carcinoma [4][5]. However, some studies have also reported that DLL3 can induce cellular apoptosis in hepatocellular carcinoma [6]. DLL3 has been found to modulate the epithelial-mesenchymal transition (EMT) process, thereby affecting the migration and invasion of cancer cells, such as in SCLC [5][6]. DLL3 is expressed in the presomitic mesoderm during embryonic development and plays a role in somite patterning and axial skeleton development [11][12].
DLL3 is highly expressed in neuroendocrine tumors, including SCLC, and is a downstream target of the transcription factor ASCL1, which is related to the neuroendocrine phenotype [1][4][7]. Due to its specific expression on the surface of neuroendocrine tumor cells, DLL3 has emerged as a promising target for antibody-drug conjugate (ADC) therapies, particularly in SCLC and other high-grade neuroendocrine tumors [8-10].
References:
[1] D. Morgensztern, B. Besse, L. Greillier, R. Santana-Dávila, N. Ready, C. Hann, et al. Efficacy and safety of rovalpituzumab tesirine in third-line and beyond patients with dll3-expressing, relapsed/refractory small-cell lung cancer: results from the phase ii trinity study, Clinical Cancer Research, vol. 25, no. 23, p. 6958-6966, 2019. https://doi.org/10.1158/1078-0432.ccr-19-1133
[2] S. Tendler, L. Kanter, R. Lewensohn, C. Ortiz-Villalón, K. Viktorsson, & L. Petris. The prognostic implications of notch1, hes1, ascl1, and dll3 protein expression in sclc patients receiving platinum-based chemotherapy, Plos One, vol. 15, no. 10, p. e0240973, 2020. https://doi.org/10.1371/journal.pone.0240973
[3] K. Matsuo, K. Taniguchi, H. Hamamoto, Y. Inomata, K. Komura, T. Tanaka, et al. Delta‐like canonical notch ligand 3 as a potential therapeutic target in malignancies: a brief overview, Cancer Science, vol. 112, no. 8, p. 2984-2992, 2021. https://doi.org/10.1111/cas.15017
[4] C. Zhu. The predictive value of delta-like3 and serum nse in evaluating chemotherapy response and prognosis in patients with advanced small cell lung carcinoma: an observational study, Medicine, vol. 103, no. 23, p. e38487, 2024. https://doi.org/10.1097/md.0000000000038487
[5] M. Furuta, H. Kikuchi, T. Shoji, Y. Takashima, E. Kikuchi, J. Kikuchi, et al. dll3 regulates the migration and invasion of small cell lung cancer by modulating snail, Cancer Science, vol. 110, no. 5, p. 1599-1608, 2019. https://doi.org/10.1111/cas.13997
[6] L. Yang, G. Wang, H. Shi, S. Jia, J. Ruan, R. Cui, et al. Methylation-driven gene dll3 is a potential prognostic biomarker in ocular melanoma correlating with metastasis, Frontiers in Oncology, vol. 12, 2022. https://doi.org/10.3389/fonc.2022.964902
[7] M. Furuta, J. Sakakibara‐Konishi, H. Kikuchi, H. Yokouchi, H. Nishihara, H. Minemura, et al. Analysis of dll3 and ascl1 in surgically resected small cell lung cancer (hot1702), The Oncologist, vol. 24, no. 11, p. e1172-e1179, 2019. https://doi.org/10.1634/theoncologist.2018-0676
[8] K. Krytska, C. Casey, J. Pogoriler, D. Martı́nez, K. Rathi, A. Farrel, et al. Evaluation of the dll3-targeting antibody–drug conjugate rovalpituzumab tesirine in preclinical models of neuroblastoma, Cancer Research Communications, vol. 2, no. 7, p. 616-623, 2022. https://doi.org/10.1158/2767-9764.crc-22-0137
[9] L. Saunders, A. Bankovich, W. Anderson, M. Aujay, S. Bheddah, K. Black, et al. A dll3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo, Science Translational Medicine, vol. 7, no. 302, 2015. https://doi.org/10.1126/scitranslmed.aac9459
[10] L. Brčić, C. Kuchler, S. Eidenhammer, D. Pabst, F. Quehenberger, A. Gazdar, et al. Comparison of four dll3 antibodies performance in high grade neuroendocrine lung tumor samples and cell cultures, Diagnostic Pathology, vol. 14, no. 1, 2019. https://doi.org/10.1186/s13000-019-0827-z
[11] I. Geffers, K. Serth, G. Chapman, R. Jaekel, K. Schuster-Gossler, R. Cordes, et al. Divergent functions and distinct localization of the notch ligands dll1 and dll3 in vivo, The Journal of Cell Biology, vol. 178, no. 3, p. 465-476, 2007. https://doi.org/10.1083/jcb.200702009
[12] P. Turnpenny, N. Whittock, J. Duncan, S. Dunwoodie, K. Kusumi, & S. Ellard. Novel mutations in dll3, a somitogenesis gene encoding a ligand for the notch signalling pathway, cause a consistent pattern of abnormal vertebral segmentation in spondylocostal dysostosis, Journal of Medical Genetics, vol. 40, no. 5, p. 333-339, 2003. https://doi.org/10.1136/jmg.40.5.333