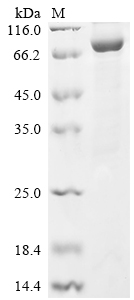
The Recombinant human Prolyl endopeptidase FAP is produced in E. coli and exhibits a purity exceeding 85%, confirmed by SDS-PAGE analysis. This partial-length protein, spanning the 26-760aa region, is tagged with an N-terminal 6xHis-tag, enhancing its utility in research applications. This recombinant FAP is available in either liquid form or as a lyophilized powder, offering flexibility in experimental design.
FAP exhibits robust endopeptidase activity, cleaving peptide bonds within proteins, while its dipeptidyl peptidase activity is relatively weak [1]. This dual functionality allows FAP to participate in various biological processes, such as the activation of growth factors and the modulation of inflammatory responses [2][3].
FAP is predominantly expressed in the reactive stroma of tumors, making it an attractive target for cancer therapies. Its expression is upregulated in various cancers, including breast, colorectal, and pancreatic cancers, where it is associated with tumor progression and metastasis [4][5]. FAP not only contributes to the degradation of extracellular matrix components but also influences the behavior of fibroblasts and other stromal cells, thereby facilitating tumor growth and invasion [6][7].
References:
[1] C. Huang, C. Suen, C. Lin, C. Chien, H. Lee, K. Chunget al., Cleavage-site specificity of prolyl endopeptidase fap investigated with a full-length protein substrate, The Journal of Biochemistry, vol. 149, no. 6, p. 685-692, 2011. https://doi.org/10.1093/jb/mvr017
[2] D. Dunshee, T. Bainbridge, N. Kljavin, J. Zavala-Solorio, A. Schroeder, R. Chanet al., Fibroblast activation protein cleaves and inactivates fibroblast growth factor 21, Journal of Biological Chemistry, vol. 291, no. 11, p. 5986-5996, 2016. https://doi.org/10.1074/jbc.m115.710582
[3] F. Keane, N. Nadvi, T. Yao, & M. Gorrell, Neuropeptide y, b‐type natriuretic peptide, substance p and peptide yy are novel substrates of fibroblast activation protein‐α, Febs Journal, vol. 278, no. 8, p. 1316-1332, 2011. https://doi.org/10.1111/j.1742-4658.2011.08051.x
[4] A. LeBeau, W. Brennen, S. Aggarwal, & S. Denmeade, Targeting the cancer stroma with a fibroblast activation protein-activated promelittin protoxin, Molecular Cancer Therapeutics, vol. 8, no. 5, p. 1378-1386, 2009. https://doi.org/10.1158/1535-7163.mct-08-1170
[5] K. Lee, K. Jackson, V. Christiansen, C. Lee, J. Chun, & P. McKee, Antiplasmin-cleaving enzyme is a soluble form of fibroblast activation protein, Blood, vol. 107, no. 4, p. 1397-1404, 2006. https://doi.org/10.1182/blood-2005-08-3452
[6] K. Chung, S. Hsu, Y. Chu, M. Lin, W. Jiaang, R. Chenet al., Fibroblast activation protein (fap) is essential for the migration of bone marrow mesenchymal stem cells through rhoa activation, Plos One, vol. 9, no. 2, p. e88772, 2014. https://doi.org/10.1371/journal.pone.0088772
[7] K. Jackson, V. Christiansen, V. Yadav, R. Silasi‐Mansat, F. Lupu, V. Awasthiet al., Suppression of tumor growth in mice by rationally designed pseudopeptide inhibitors of fibroblast activation protein and prolyl oligopeptidase, Neoplasia, vol. 17, no. 1, p. 43-54, 2015. https://doi.org/10.1016/j.neo.2014.11.002