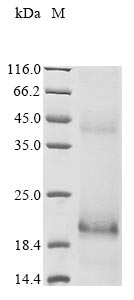
Recombinant Human papillomavirus 11 Protein E7 is expressed in E. coli and covers the complete protein sequence from amino acids 1-98. The protein carries an N-terminal 10xHis-tag and a C-terminal Myc-tag, which helps with both purification and detection processes. SDS-PAGE analysis shows the protein achieves greater than 90% purity, which appears to support reliable performance in research settings. This protein is for research use only.
HPV11 Protein E7 plays what seems to be a central role in studying how viruses interact with their host cells. E7 disrupts normal cellular control mechanisms, particularly those involved in cell cycle regulation. Its ability to alter host cell functions makes it an important research target for scientists trying to understand viral disease development and explore possible treatments.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Human papillomavirus 11 Protein E7 is an oncoprotein that requires precise folding, proper zinc-binding domain formation (via two CXXC motifs), and specific tertiary structure for its functional activity in cell transformation and binding to host proteins like Rb. The E. coli expression system cannot provide the eukaryotic folding environment, and more critically, cannot support the zinc incorporation essential for E7's structural stability and function. The dual N-terminal 10xHis-tag and C-terminal Myc-tag may cause steric interference with the protein's functional domains. While the full-length protein (1-98aa) contains all functional residues, the probability of correct folding with functional bioactivity is extremely low without experimental validation of zinc incorporation and proper tertiary structure.
1. Antibody Development and Validation
This application is highly suitable as antibody development relies on antigenic sequence recognition rather than functional protein folding. The full-length protein provides comprehensive epitope coverage for generating E7-specific antibodies. However, antibodies may not recognize conformational epitopes of the native, zinc-bound E7 protein.
2. Biochemical Characterization and Structural Studies
Basic biophysical analysis can be performed, but will not reflect native E7 structure. Techniques like circular dichroism spectroscopy may assess secondary structure, but the lack of zinc incorporation means the protein will not adopt its native conformation. Results will describe an artificial construct rather than the physiological oncoprotein.
3. ELISA-Based Detection Assay Development
This application is feasible but has limitations. ELISA development relies on antigenic recognition, but the misfolded protein may not present native conformational epitopes, potentially reducing assay sensitivity for detecting antibodies against physiological E7.
Final Recommendation & Action Plan
This E. coli-expressed HPV11 E7 with dual tags is unsuitable for functional studies due to the essential requirement for zinc incorporation that cannot be met in this expression system. The protein will lack the proper zinc-binding and tertiary structure necessary for authentic oncoprotein function. Applications 1 and 3 can proceed, but with awareness that they will primarily target linear epitopes. Application 2 provides only a basic characterization of the misfolded protein. For reliable E7 research, use mammalian-expressed protein with verified zinc incorporation or implement refolding protocols with zinc supplementation followed by rigorous functional validation.