Bacterial antigens are crucial in developing effective vaccines, serving as key components that stimulate the immune response. Understanding the specific antigens associated with pathogenic bacteria is essential for designing targeted vaccines to prevent infections. What exactly are these antigens? And how are they selected for vaccines?
Table of Contents
1. What are Bacterial Antigens?
Bacterial antigens are molecules on the surface or inside of bacteria that can be recognized by the host immune system and trigger an immune response. They play a significant role in the pathogenesis of bacterial infections and are thus crucial for the development of vaccines.
2. Common Bacterial Antigens Used in Vaccines
Bacterial antigens commonly used in vaccine development can be classified into various types based on their structure and function, including protein antigens, polysaccharide antigens, and toxins.
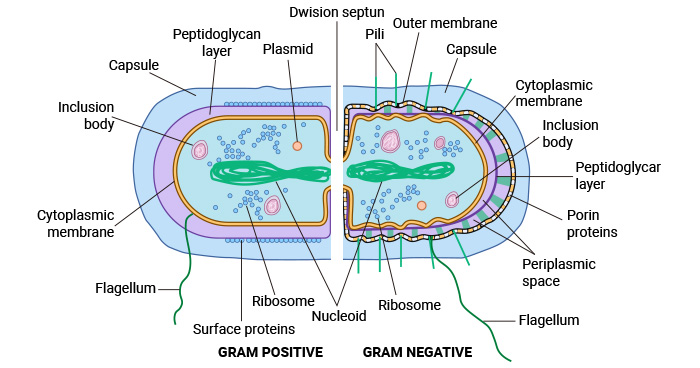
Figure 1. Bacterial Cell Structure and Organization
This picture is cited from: https://www.sciencedirect.com/science/article/abs/pii/B9780128187319001919?via%3Dihub
2.1 Protein Antigens
Protein antigens are the most common type used in bacterial vaccines. These include surface proteins, flagellar proteins (H antigens), pili (fimbrial antigens), and enzymes produced by the bacteria. Protein-based antigens are highly immunogenic and can induce a strong and specific immune response. Some commonly used protein antigens in vaccines include pilus proteins in Streptococcus pneumoniae, fimbriae proteins in Escherichia coli, and outer membrane proteins (OMPs) in Neisseria meningitidis.
These protein antigens can be whole proteins, fragmented peptides, subunits, or recombinant proteins that represent the most immunogenic regions of the bacterial protein.
2.2 Polysaccharide (K) Antigens
Bacterial capsular polysaccharides are found on the outer surface of bacteria and constitute the primary antigens in most pathogenic bacteria [1]. O antigens, the outermost part of the lipopolysaccharide (LPS) in Gram-negative bacteria, play a significant role in immune evasion and can serve as targets for vaccine development [2].
While polysaccharide antigens do not efficiently elicit T cell help for antibody generation, particularly in infants. To overcome this limitation, polysaccharide antigens are often conjugated to proteins to enhance immunogenicity, forming polysaccharide-protein conjugate vaccines.
Examples of vaccines using protein-polysaccharide conjugates include Haemophilus influenzae type B vaccine, Streptococcus pneumoniae (pneumococcal) vaccine, and Neisseria meningitidis (meningococcal) vaccine.
2.3 Toxins
Bacterial toxins are harmful substances secreted by bacteria, and many vaccines are developed to neutralize these toxins. Toxins, such as those produced by Corynebacterium diphtheriae (diphtheria toxin) or Clostridium tetani (tetanus toxin), are chemically inactivated to form "toxoids," which are safe and used as antigens in tetanus vaccine and diphtheria vaccine, respectively.
Toxoid vaccines elicit the production of neutralizing antibodies, which can rapidly bind and neutralize bacterial toxins before they cause infection.
3. The Role of Bacterial Antigens in Vaccines
Bacterial vaccines train the immune system by simulating an infection. The bacterial antigens in the vaccine are recognized and remembered by immune cells, allowing them to respond quickly when a real infection occurs.
Bacterial antigens in the vaccines protect against bacterial infection by stimulating both innate and adaptive immunity in the host. Adaptive immunity includes T lymphocyte-mediated cellular immunity and B lymphocyte-mediated humoral immunity, which are closely associated with antigen-presenting cells and promote the specific elimination of bacterial pathogens.
3.1 Recognition by Innate Immunity
When the vaccine containing bacterial antigens is administered, bacterial antigens are recognized by the innate immune system, the body's first line of defense. This recognition is primarily mediated by pattern recognition receptors (PRRs) such as TLRs, which detect pathogen-associated molecular patterns (PAMPs) present on bacterial antigens including peptidoglycan, lipopolysaccharide (LPS), flagellin, and teichoic acids [3].
Upon recognition of these antigens, immune cells such as macrophages, dendritic cells, and neutrophils become activated and initiate an inflammatory response. This includes the secretion of cytokines, recruitment of additional immune cells, and phagocytosis of the bacteria [4].
CD14, a membrane receptor for bacterial LPS, binds to LPS binding protein (LBP) and then interacts with Gram-negative bacteria LPS, forming the triple complex of LPS-LBP-CD14, which subsequently binds to TLR4 with the help of MD-2, activating a cascade of intracellular signaling, including NF-κB and MAPK pathways. This leads to the production of pro-inflammatory cytokines like TNF-α and IL-6, which amplify the immune response [5].
3.2 T Cell-Mediated Immune Response to Bacterial Antigens
Once innate immune cells like dendritic cells phagocytose bacteria, they process the bacterial antigens and present them to T cells through major histocompatibility complex (MHC) molecules [6].
MHC class II molecules present bacterial antigens to CD4+ helper T cells, which is crucial for coordinating the adaptive immune response. MHC class I molecules can present bacterial antigens to CD8+ cytotoxic T cells, especially for intracellular bacteria, which leads to the killing of infected cells.
3.3 Humoral Immune Response to Bacterial Antigens
Activated helper T cells release cytokines that stimulate B cells to produce antibodies against specific bacterial antigens. Bacterial antigens, particularly capsular polysaccharides, LPS, and exotoxins, can induce B cell activation and the production of specific antibodies.
Antibodies can neutralize toxins or prevent bacteria from attaching to and infecting host cells (called neutralization). Or antibodies coat bacteria, making them more easily recognized and engulfed by phagocytic cells like macrophages and neutrophils (called opsonization). Certain antibodies especially IgG and IgM, activate the complement system, which leads to bacterial lysis or enhances phagocytosis (called complement activation).
3.4 Immunological Memory Formation
Bacterial antigens also play a role in generating immunological memory, which is crucial for long-term protection against subsequent infections.
Following vaccination, memory B cells and memory T cells specific to bacterial antigens are formed. These cells persist in the body and allow for a faster and more robust immune response upon re-exposure to the same bacterium [7].
During the immune response, T cells and other immune cells release a variety of cytokines, which play a crucial role in coordinating the immune response, thus promoting inflammation, recruiting more immune cells, and boosting the activity of both T cells and B cells.
4. Selection and Development of Bacterial Antigens for Vaccine Candidates
The selection and development of bacterial antigens for vaccine candidates uses various methodologies, including reverse vaccinology, immunoproteomics, and the identification of surface-exposed proteins. The integration of these approaches allows for the systematic identification of antigens that can elicit robust immune responses.
4.1 Antigen Selection Criteria
Choosing the right bacterial antigen for a vaccine involves several key criteria: immunogenicity, pathogen specificity, stability, and conservation across most strains.
- Immunogenicity: The bacterial antigen must be capable of stimulating a strong immune response.
- Pathogen specificity: The bacterial antigen should be unique to the pathogen and not cross-react with human tissues or other non-harmful bacteria.
- Stability: The bacterial antigen must remain stable in its vaccine formulation without losing its immunogenic properties.
- Conservation across strains: In the case of bacteria with multiple strains, the antigen should be conserved across the most pathogenic strains to ensure broad protection.
4.2 Methodologies Used in Bacterial Antigen Discovery
Reverse vaccinology is an innovative approach that uses bioinformatics and genome sequencing to identify potential vaccine antigens. By analyzing the genetic makeup of a pathogen, researchers can predict proteins that are likely to be surface-expressed or secreted by the bacteria, which may serve as effective antigens. This method typically focuses on selecting proteins that are conserved across strains, surface-exposed, and capable of eliciting an immune response [8].
Studies have shown that reverse vaccinology can effectively identify protective antigens from various bacterial species, including Neisseria meningitidis and Acinetobacter baumannii [9,10].
Immunoproteomics complements reverse vaccinology by providing empirical data on the immunogenicity of identified antigens. This technique involves the use of serum from infected or vaccinated individuals to identify antigens that elicit an immune response [11]. Immunoproteomic studies have successfully identified antigens from respiratory pathogens [11]. The combination of immunoproteomics with reverse vaccinology enhances the reliability of antigen selection by validating computational predictions with experimental evidence [12].
The identification of bacterial surface antigens is crucial, as these proteins are often the first point of contact with the host immune system. Techniques such as peptide phage display have been employed to screen for surface antigens, leading to the identification of several promising candidates for vaccine development [13].
Conclusion and Outlook
Bacterial antigens play a pivotal role in the development of effective vaccines by enabling the immune system to recognize and combat bacterial pathogens. By utilizing protein antigens, polysaccharides, and inactivated toxins, vaccine developers have created life-saving vaccines that prevent diseases such as pneumococcal infections, diphtheria, tetanus, and pertussis. However, challenges remain, especially in developing vaccines for pathogens like Staphylococcus aureus and Mycobacterium tuberculosis.
Future research will likely focus on integrating emerging technologies such as nanotechnology and synthetic biology with traditional vaccine approaches, enhancing both the safety and efficacy of bacterial vaccines. Continuous innovation in antigen selection, vaccine formulation, and delivery methods will ensure that bacterial vaccines remain a cornerstone of global public health for generations to come.
CUSABIO can provide some recombinant bacterial antigens for vaccine research studies, including bacterial toxins, bacterial fimbrial (or flagellar) antigens, and bacterial protein antigens.
References
[1] U. Nwodo, E. Green, & A. Oko. Bacterial exopolysaccharides: functionality and prospects [J]. International Journal of Molecular Sciences, vol. 13, no. 11, p. 14002-14015, 2012.
[2] C. Chaput, E. Spindler, R. Gill, & A. Zychlinsky. O-antigen protects gram-negative bacteria from histone killing [J]. Plos One, vol. 8, no. 8, p. e71097, 2013.
[3] Janeaway, C.A. Jr. & Medzhitov, R. Innate immune recognition [J]. Annu. Rev. Immunol. 20, 197–216 (2002).
[4] J. Fertey, L. Bayer, et al. Low-energy electron irradiation efficiently inactivates the gram-negative pathogen rodentibacter pneumotropicus—a new method for the generation of bacterial vaccines with increased efficacy [J]. Vaccines, vol. 8, no. 1, p. 113, 2020.
[5] Park, B., Lee, JO. Recognition of lipopolysaccharide pattern by TLR4 complexes [J]. Exp Mol Med 45, e66 (2013).
[6] Blander, J. M. & Medzhitov, R. Toll-dependent selection of microbial antigens for presentation by dendritic cells [J]. Nature 440, 808–812 (2006).
[7] A. Mayer, V. Balasubramanian, A. Walczak, & T. Mora. How a well-adapting immune system remembers [J]. Proceedings of the National Academy of Sciences, vol. 116, no. 18, p. 8815-8823, 2019.
[8] A. D’Mello, C. Ahearn, T. Murphy, & H. Tettelin. Revac: a reverse vaccinology computational pipeline for prioritization of prokaryotic protein vaccine candidates [J]. BMC Genomics, vol. 20, no. 1, 2019.
[9] P. Baliga, M. Shekar, & M. Venugopal. Potential outer membrane protein candidates for vaccine development against the pathogen vibrio anguillarum: a reverse vaccinology based identification [J]. Current Microbiology, vol. 75, no. 3, p. 368-377, 2017.
[10] M. Chiang, W. Sung, et al. Identification of novel vaccine candidates againstacinetobacter baumanniiusing reverse vaccinology [J]. Human Vaccines & Immunotherapeutics, vol. 11, no. 4, p. 1065-1073, 2015.
[11] R. Dennehy and S. McClean. Immunoproteomics: the key to discovery of new vaccine antigens against bacterial respiratory infections [J]. Current Protein and Peptide Science, vol. 13, no. 8, p. 807-815, 2012.
[12] M. Dalsass, A. Brozzi, D. Medini, & R. Rappuoli. Comparison of open-source reverse vaccinology programs for bacterial vaccine antigen discovery [J]. Frontiers in Immunology, vol. 10, 2019.
[13] Y. Hu, D. Zhao, et al. Identification of bacterial surface antigens by screening peptide phage libraries using whole bacteria cell-purified antisera [J]. Frontiers in Microbiology, vol. 8, 2017.
CUSABIO team. What Bacterial Antigens Are Used in Vaccines?. https://www.cusabio.com/c-21193.html



Comments
Leave a Comment