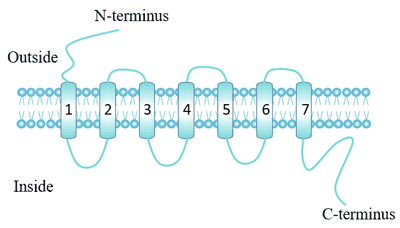
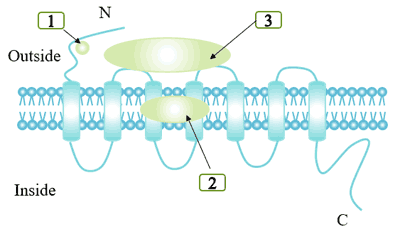
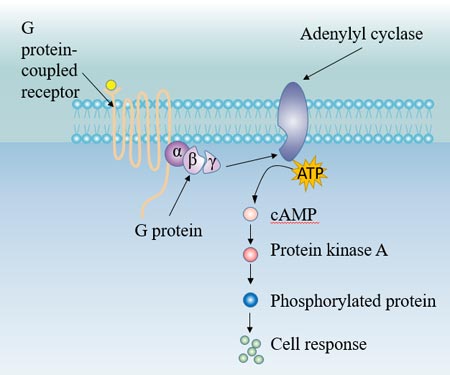
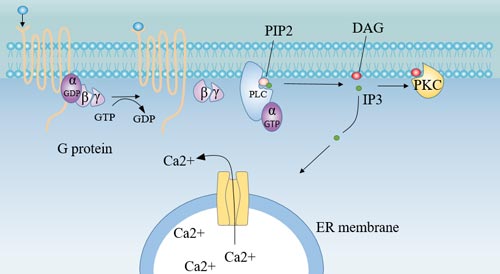
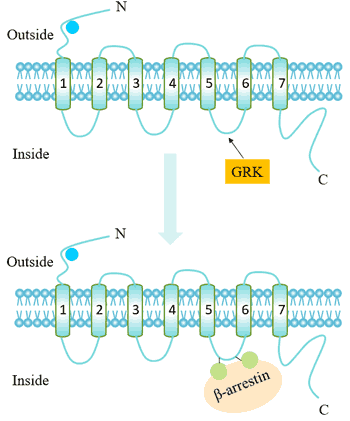
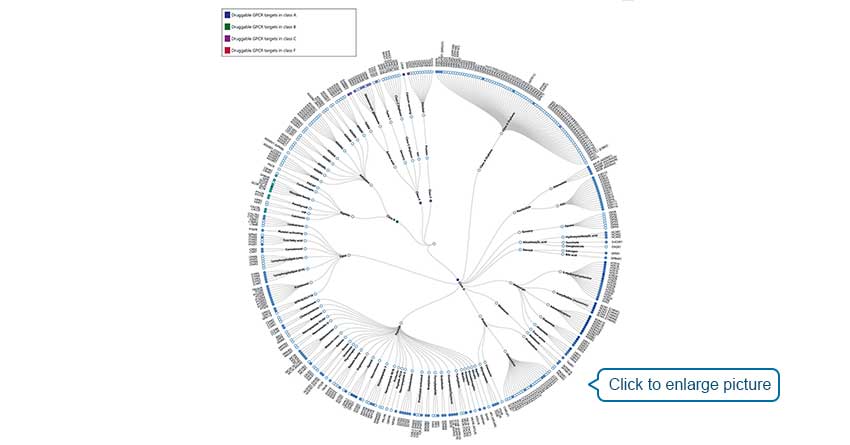
[1] Strader C D, Fong T M, Tota M R, et al. Structure and Function of G Protein-Coupled Receptors [J]. Annual Review of Biochemistry, 1994, 63(1): 101-132.
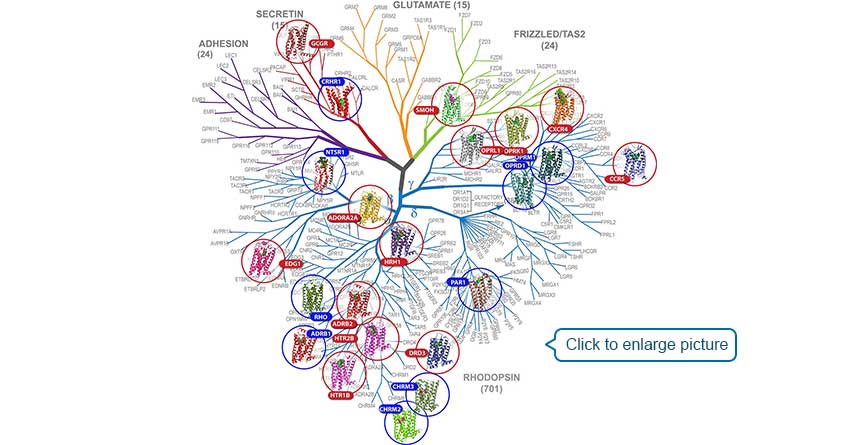
[2] Kolakowski L, Kolakowski L J, Kolakowski L F. GCRDb: a G-protein-coupled receptor database [J]. Receptors Channels, 1994, 2(1): 1-7.
[3] Meunier J C, Mollereau C, Toll L, et al. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor [J]. Nature (London), 1995, 377(6549): 532-535.
[4] Reinscheid R K, Nothacker H P, Bourson A, et al. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor [J]. Science, 1995, 270(5237): 792-794.
[5] Misra S, Wu Y, Venkataraman G, et al. Heterotrimeric G - protein complex and G-protein coupled receptor from a legume (Pisum sativum): role in salinity and heat stress and cross-talk with phospholipase C [J]. Plant Journal, 2010, 51(4): 656-669.
[6] Gookin T E, Kim J, Assmann S M. Whole proteome identification of plant candidate G-protein coupled receptors in Arabidopsis, rice, and poplar: computational prediction and in-vivo protein coupling [J]. Genome Biology, 2008, 9(7): R120-R120.
[7] Gabriella C, Fabio A, Nicole A, et al. GCR1, the putative Arabidopsis G protein-coupled receptor gene is cell cycle-regulated, and its overexpression abolishes seed dormancy and shortens time to flowering [J]. Proceedings of the National Academy of Sciences of the United States of America, 2002, 99(7): 4736-4741.
[8] Pandey S, Assmann S M. The Arabidopsis Putative G Protein-Coupled Receptor GCR1 Interacts with the G Protein α Subunit GPA1 and Regulates Abscisic Acid Signaling [J]. Plant Cell, 2004, 16(6): 1616-1632.
[9] Warpeha K M, Upadhyay S, Yeh J, et al. The GCR1, GPA1, PRN1, NF-Y Signal Chain Mediates Both Blue Light and Abscisic Acid Responses in Arabidopsis [J]. Plant Physiology, 2007, 143(4): 1590-1600.
[10] Liu X, Yue Y, Li B, et al. A G Protein-Coupled Receptor Is a Plasma Membrane Receptor for the Plant Hormone Abscisic Acid [J]. Science, 315.
[11] Santner A, Estelle M. Recent advances and emerging trends in plant hormone signaling [J]. Nature, 2009, 459(7250): 1071-8.
[12] Plakidou-Dymock S, Dymock D, Hooley R. A higher plant seven-transmembrane receptor that influences sensitivity to cytokinins [J]. Current Biology, 1998, 8(6): 315-324.
[13] Apone F, Alyeshmerni N, Wiens K, et al. The G-Protein-Coupled Receptor GCR1 Regulates DNA Synthesis through Activation of Phosphatidylinositol-Specific Phospholipase C [J]. Plant Physiology, 2003, 133(2): 571-579.
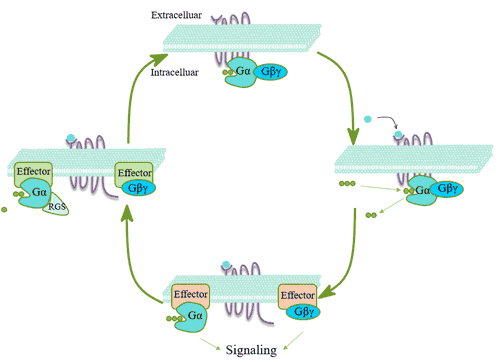
[14] Hamm, H. E. The Many Faces of G Protein Signaling [J]. Journal of Biological Chemistry, 1998, 273(2): 669-672.
[15] Logothetis D E, Kurachi Y, Galper J, et al. The βγ subunits of GTP-binding proteins activate the muscarinic K+ channel in heart [J]. Nature, 1987, 325(6102): 321-326.
[16] Ford C, Skiba N P, Bae H, et al. Molecular basis for interactions of G protein beta gamma subunits with effectors [J]. Science, 1998, 280(5367): 1271-1274.
[17] Rondard P, Iiri T, Srinivasan S, et al. Mutant G protein alpha subunit activated by G beta gamma: a model for receptor activation? [J]. Proc Natl Acad Sci USA, 2001, 98(11): 6150-6155.
[18] Chuang D M, Costa E. Evidence for internalization of the recognition site of beta-adrenergic receptors during receptor subsensitivity induced by (-)-isoproterenol [J]. Proceedings of the National Academy of Sciences, 1979, 76(6): 3024-3028.
[19] Skolnick J, Gao M, Roy A, et al. Implications of the small number of distinct ligand binding pockets in proteins for drug discovery, evolution and biochemical function [J]. Bioorganic & Medicinal Chemistry Letters, 2015, 25(6): 1163-1170.
[20] MartíSolano M, Schmidt D, Kolb P, et al. Drugging specific conformational states of GPCRs: challenges and opportunities for computational chemistry [J]. Drug Discovery Today, 2016, 21(4): 625-631.
[21] Guo Z, Li B, Cheng L T, et al. Identification of Protein-Ligand Binding Sites by the Level-Set Variational Implicit-Solvent Approach [J]. Journal of Chemical Theory and Computation, 2015, 11(2): 753-765.
[22] Thathiah A, Strooper B D. The role of G protein-coupled receptors in the pathology of Alzheimer's disease [J]. Nature Reviews Neuroscience, 2011, 12(2): 73-87.
[23] Huang J, Lakkaraju S K, Coop A, et al. Conformational Heterogeneity of Intracellular Loop 3 of the μ-Opioid G-Protein Coupled Receptor [J]. The Journal of Physical Chemistry B, 2016: acs.jpcb.6b09351.
[24] Hauser A S, Chavali S, Masuho I, et al. Pharmacogenomics of GPCR Drug Targets [J]. Cell, 2017, 172(1-2): 41-54.
[25] Marinissen M J, Gutkind J S. G-protein-coupled receptors and signaling networks: emerging paradigms [J]. Trends in Pharmacological Sciences, 2001, 22(7): 0-376.
[26] Yang D H, Zhou Q T, Labroska V, et al. G protein-coupled receptors: structure- and function-based drug discovery[J]. Signal Transduction and Targeted Therapy, 2021, 6(1) : 7-7.
[27] https://phys.org/news/2013-12-x-ray-laser-slac-important-drug.html