What Is Glutamatergic Synapse?
Synapses are important structures that connect neurons in the nervous system through chemical or electrical signals. Glutamatergic synapses are the main excitatory synapses in the brain. These synapses consist of glutamate localized inside presynaptic vesicles and glutamate receptors on the postsynaptic membrane.
The Function of Glutamatergic Synapse
Glutamatergic synapse is involved in regulating the establishment of neural network connections during the brain and spinal cord development and mediating the cellular processes pivotal for synaptic transmission and plasticity. The correct functioning of glutamatergic synapses is essential for learning and memory. Glutamate released from presynaptic vesicle has an important effect on modulating neuronal growth & survival and synaptic connections, so disruptions of glutamate homeostasis may play a relevant role in neurological/neurodegenerative diseases.
The Signaling pathway of Glutamatergic Synapse
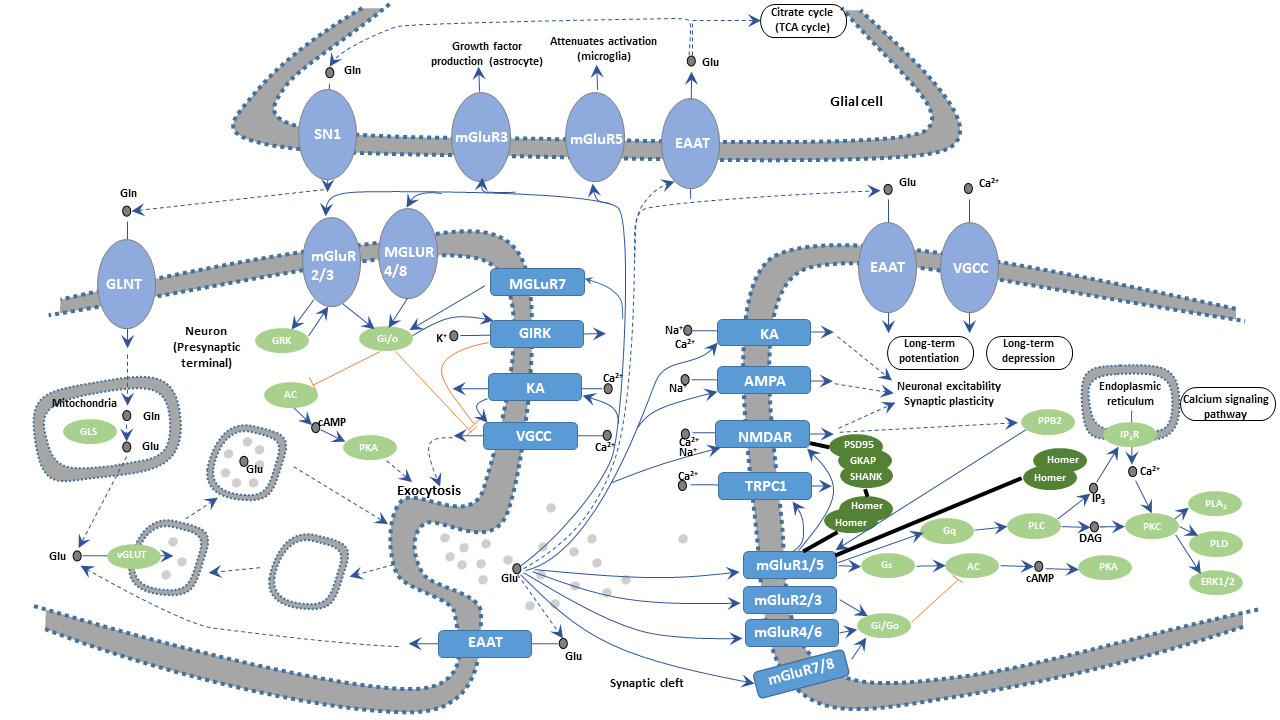
Glutamate (Glu) is the most abundant amino acids in the mammalian brain, with the highest content in the cerebral cortex and hippocampus. About 40 percent of synapses use glutamate as a transmitter. The glutamate is taken into astrocytes by the sodium ion-dependent excitatory amino acid transporter (EAAT) located on the membranes of astrocytes. The captured glutamate is converted into glutamine (GIn) by the glutamine synthetase and then reenters the presynaptic neuron terminals. GIn generates Glu through glutamine deamination. Part of glutamine is catalyzed by glutamate decarboxylase to produce inhibitory GABA. Glu is translocated into the synaptic vesicle by EAATs on the synaptic vesicle and stored in these vesicles.
During synaptic transmission, nerve impulses are transmitted to the presynaptic terminal, depolarizing the presynaptic membrane, which leads to the influx of calcium ions (Ca2+) into the presynapse due to the opening of the voltage-gated calcium channel (VGCC). Glutamate is released into the synaptic cleft through the fusion of synaptic vesicles and presynaptic membranes at the active zone. Released Glu acts on various types of Glu receptors (GluRs) in the postsynaptic membrane, transmitting nerve impulses and exerting multiple physiological roles.
Glutamate receptors are numerous and highly complex. These transmembrane protein receptors are located in both neurons and glial cells throughout the central nervous system (CNS). There are more than 20 glutamate receptors found in the mammalian central nervous system. They fall into two main categories, ionotropic glutamate receptors (iGluRs) and metabotropic glutamate receptors (mGluRs). The former are voltage-sensitive glutamate-gated ion channels, whereas the latter are ligand-sensitive G protein-coupled receptors (GPCRs)activated by glutamate. IGluRs can be further subdivided into N-methyl-D-aspartate (NMDA) receptors, α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors, and Kainate (KA) receptors.
Glutamate binds to NMDA receptors, leading to the sodium and potassium ion permeability of the two sides of the postsynaptic membrane increases, which produces excitatory postsynaptic potential (EPSP). Calcium ions also flood into the post neuron cell due to increased calcium ion permeability. Calcium ion acts as a second messenger to activate calcium-dependent enzymes such as Ca2+/calmodulin-dependent protein kinases, inducing changes in intracellular physiological effects. During synaptic transmission, the activation of NMDA receptors requires the involvement of non-NMDA receptors that mainly refer to AMPA receptors. The interaction between AMPA receptor and glutamate promotes local depolarization of the postsynaptic membrane of adjacent NMDA receptors. In general, NMDA receptors cause a relatively slow and sustained EPSP. The role of the NMDA receptor and Glu is closely related to synaptic plasticity, learning, and memory.
The mGluRs bind glutamate within a large extracellular domain and transmit signals through the receptor protein to intracellular molecules. In the mGluR-mediated signaling pathway, glutamate regulates cell excitability and synaptic transmission via the second messenger signaling pathways such as PLC pathway, cAMP pathway, and PI3K-Akt pathway.
Appropriate stimulation of GluRs is necessary for learning and memory. But excessive activation of GluRs in the hippocampus region produces pathological long-term potentiation (LTP) and may even cause excitotoxicity that will impair cognition and induce neuronal damage & death.





