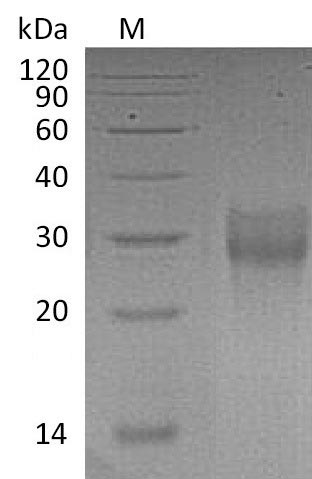
Recombinant Human Tumor necrosis factor receptor superfamily member 9 (TNFRSF9) is produced in a mammalian expression system, ensuring proper folding and post-translational modifications. The extracellular domain (24-186aa) is expressed with a C-terminal 6xHis tag for ease of purification and detection. The protein is highly pure, with a purity greater than 95% by SDS-PAGE, and exhibits biological activity, binding Anti-Human CD137 mAb-Fc with an affinity constant of 25.5 nM. Endotoxin levels are controlled to less than 1.0 EU/µg.
TNFRSF9, also known as 4-1BB, belongs to the tumor necrosis factor receptor superfamily. It appears to play a critical role in immune system function by providing co-stimulatory signals that T cells need for activation and survival. This protein is involved in immune regulation and has become a focus in research related to immune responses and potential therapeutic targets for boosting immune activity in various diseases.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
1. Antibody Development and Characterization Studies
This recombinant TNFRSF9 (CD137) extracellular domain is confirmed to be antigenically active (25.5 nM affinity to anti-CD137 mAb) and suitable for antibody development. However, the C-terminal His-tag may induce tag-specific antibodies, potentially reducing antibodies against native C-terminal epitopes. While mammalian expression ensures proper folding and glycosylation, comprehensive validation should include testing against full-length, membrane-associated CD137 to ensure recognition of physiological receptor forms. The high purity supports immunization protocols.
2. Binding Kinetics and Interaction Studies
The protein is appropriate for binding studies, demonstrating good affinity (25.5 nM) for the anti-CD137 antibody. However, the C-terminal His-tag may cause minor steric effects near the binding interface, potentially affecting precise kinetic measurements. While BLI validation confirms functionality, researchers should validate binding parameters with tag-free CD137 for absolute accuracy, particularly for therapeutic antibody development requiring precise affinity measurements.
3. Biochemical Assays and Functional Studies
The protein is suitable for ligand-binding assays, but as an extracellular domain fragment, it cannot model full receptor signaling requiring transmembrane and intracellular domains. While useful for studying initial binding events, researchers cannot investigate downstream NF-κB signaling or costimulatory function with this soluble fragment. Functional studies should be complemented with full-length CD137 expressed in cellular systems.
4. Structural Biology and Protein Engineering Research
The extracellular domain is valuable for structural studies, but the C-terminal His-tag may impede crystallization and require cleavage for high-resolution structural work. The mammalian glycosylation ensures native-like folding but may introduce heterogeneity for crystallization. This construct is suitable for studying the ligand-binding interface but cannot inform about the full receptor architecture, including transmembrane organization.
5. Immunoassay Development and Validation
This protein is excellent for immunoassay development, with the His-tag facilitating immobilization and the demonstrated affinity ensuring reliable detection. However, assays may only detect the extracellular domain and miss cleaved soluble CD137 forms with different biological activities. Researchers should validate assays against clinical samples containing naturally occurring soluble CD137 variants.
Final Recommendation & Action Plan
This mammalian-expressed human TNFRSF9 extracellular domain (24-186aa) with C-terminal His-tag is a well-characterized reagent suitable for most proposed applications, with particular strength in antibody development and immunoassays. For immediate use: employ the established 25.5 nM affinity as a benchmark for binding studies, but consider tag cleavage for precise kinetic measurements. For antibody development, this protein is ideal for generating extracellular domain-specific antibodies, but validated against full-length CD137 for therapeutic applications. For functional studies, use this protein for initial binding characterization, but transition to full-length receptor cellular assays for signaling investigation. The mammalian expression ensures proper glycosylation, which is critical for native antigen presentation. For structural work, the protein requires tag removal and may need glycosylation engineering for crystallization success. Always include appropriate controls for tag-related artifacts, and note that this extracellular domain cannot model the receptor clustering and signaling functions of full-length membrane-bound CD137.