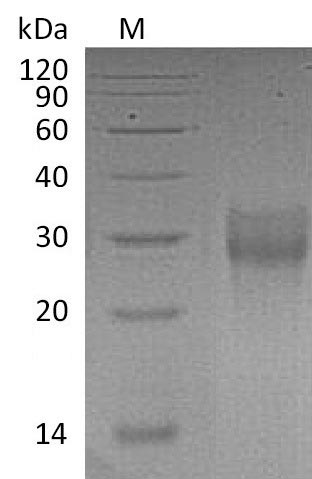
Recombinant Human Tumor necrosis factor receptor superfamily member 9 (TNFRSF9) is produced in a mammalian expression system, ensuring proper folding and post-translational modifications. The extracellular domain (24-186aa) is expressed with a C-terminal 6xHis tag for ease of purification and detection. The protein is highly pure, with a purity greater than 95% by SDS-PAGE, and exhibits biological activity, binding Anti-Human CD137 mAb-Fc with an affinity constant of 25.5 nM. Endotoxin levels are controlled to less than 1.0 EU/µg.
TNFRSF9, also known as 4-1BB, belongs to the tumor necrosis factor receptor superfamily. It appears to play a critical role in immune system function by providing co-stimulatory signals that T cells need for activation and survival. This protein is involved in immune regulation and has become a focus in research related to immune responses and potential therapeutic targets for boosting immune activity in various diseases.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
1. Antibody Development and Characterization Studies
This recombinant TNFRSF9 extracellular domain may serve as an immunogen or screening target for developing anti-CD137 antibodies. The high purity (>95%) and low endotoxin levels suggest it could work well in immunization protocols with research animals. That C-terminal His-tag makes purification and immobilization straightforward for antibody screening assays. Scientists could use this protein to evaluate binding kinetics, map epitopes, and characterize the function of newly developed anti-CD137 antibodies.
2. Binding Kinetics and Interaction Studies
Given the demonstrated biological activity and established binding affinity (25.5 nM) to Anti-Human CD137 mAb-Fc, this protein appears valuable for comparative binding studies. Bio-layer interferometry (BLI) or surface plasmon resonance (SPR) could help characterize interactions between TNFRSF9 and various ligands or therapeutic candidates. The His-tag allows for oriented immobilization on biosensor surfaces, which should provide consistent and reproducible binding measurements. This application might be particularly useful for structure-activity relationship studies and lead compound optimization.
3. Biochemical Assays and Functional Studies
The mammalian expression system helps ensure proper protein folding and post-translational modifications, making this recombinant protein suitable for biochemical assays investigating TNFRSF9 function. Scientists can use this protein in cell-based assays to study receptor-ligand interactions and downstream signaling pathways. The biological activity confirmation through BLI assay validates its utility for functional studies, though the low endotoxin content ensures minimal interference in cell culture-based experiments.
4. Structural Biology and Protein Engineering Research
This well-characterized extracellular domain (24-186aa) appears to provide a stable platform for structural studies of the TNFRSF9 receptor family. High purity and the His-tag should make crystallization trials and NMR studies more manageable when trying to understand structural features important for ligand binding. Scientists might use this protein as a template for rational protein design or as a control in comparative structural studies. The confirmed biological activity serves as a functional readout for evaluating engineered variants or mutants.
5. Immunoassay Development and Validation
The C-terminal His-tag and high purity make this protein well-suited for developing sandwich ELISAs, competitive binding assays, or other immunoassay formats. It could work as a capture antigen or standard in quantitative assays for measuring soluble TNFRSF9 levels in biological samples. Low endotoxin content and mammalian expression should ensure compatibility with sensitive detection systems, while the established binding affinity data provides a reference point for assay validation and quality control.