Histone Acetylation
Vincent Allfrey and his team first reported histone acetylation in 1964 [1]. Histone acetylation stands out as a extensively studied post-transcriptional modification (PTM) of histones, playing a central role in the dynamic modulation of chromatin-based gene transcription.
1. What Is Histone Acetylation?
Histone acetylation refers to the process of adding an acetyl group to the lysine residues on the N-terminal tail of histone proteins [1]. Negative charges of added acetyl groups neutralize lysines' positive charges, which results in a weakened histone-DNA interaction, leading to a more relaxed chromatin structure that facilitates the access of transcription factors to genomic sequences, thus increasing gene expression.
Histone acetylation has been detected on various histone proteins. Specifically, H3 displays acetylation on residues K4, K9, K14, K18, K23, K27, K36, and K56, while H4 showcases acetylation on K5, K8, K12, K16, K20, and K91. Additionally, H2A and H2B also undergo lysine acetylation, with H2A being modified at K5 and K9, and H2B experiencing acetylation at K5, K12, K15, K16, K20, and K120.
2. Enzymes or Proteins Involved in Histone Acetylation
Histone acetylation is a dynamic and reversible process synergistically regulated by histone acetylation modulators (HAMs) including writers (histone acetyltransferases), readers, and erasers (histone deacetylases) that add, recognize, and remove the acetyl group, respectively. This dynamic interplay between HATs and HDACs regulates the balance of histone acetylation, ensuring precise control of gene expression patterns.
2.1 Histone Acetyltransferases (HATs)
HATs, also called "writers", are responsible for transferring an acetyl group (-COCH3) from acetyl CoA to the ε-amino group of target lysine residues of H3 or H4 [2]. HATS also acetylate various non-histone substrates, thus they are generally categorized as lysine acetyltransferases (KATs).
HATs are classified into two major categories based on their cellular localization: type A HATs, which are located in the nucleus and acetylate nucleosomal histones and other chromatin-associated proteins, and type B HATs, which are situated in the cytoplasm and specifically acetylate free newly synthesized histones, having no direct impact on transcription.
| HAT Family |
Members |
Localization |
Histone Substrates |
Function |
| Type A HATs |
GNAT |
KAT2A (GCN5) |
Nucleus |
H3K9, H3K14, H3K18, H2B |
Transcription activation |
| KAT2B (PCAF) |
Nucleus |
H3K9, H3K14, H3K18, H2B |
Transcription activation |
| MYST |
KAT5 (Tip60) |
Nucleus |
H4K5, H4K8, H4K12, H4K16 |
Transcription activation, DNA repair |
| KAT6A (MOZ/MYST3) |
Nucleus |
histone H2B, H3K14 and H4K5, H4K8, H4K12 and H4K16 in vitro and H3K9 in vivo [3] |
Transcription activation |
| KAT6B (MORF/MYST4) |
Nucleus |
particularly H3 and H4, and nucleosomal histones with a preference for H4 |
Transcription activation |
| KAT7 (HBO1/MYST2) |
Nucleus |
H3K14, H4K5, H4K8, H4K12 |
Transcription, DNA replication |
| KAT8 (MOF/MYST1) |
Nucleus |
specifically H4K16 |
Chromatin boundaries, dosage compensation, DNA repair |
| P300/CBP |
KAT3B (P300) |
Nucleus |
all four histone subtypes: H2AK5, H2B (K5, K12, K15, K20), H3 (K14, K18, K27), and H4 (K8, K12) |
Transcription activation |
| KAT3A (CBP) |
Nucleus |
| Others |
Transcription co-activators |
KAT4 ( TAF1/TBP) |
Nucleus |
H3 and H4, H3K14 |
Transcription activation |
| KAT12 (TIFIIIC90) |
Nucleus |
H3K9, H3K14, H3K18 |
Transcription activation |
| Steroid receptor co-activators |
ATAT1
|
Nucleus |
- |
- |
| KAT13A (SRC1) |
Nucleus |
H3, H4 |
Transcription activation |
| KAT13B (SCR3/AIB1/ ACTR) |
Nucleus |
| KAT13C ( p160) |
Nucleus |
| KAT13D (CLOCK)
|
Nucleus |
| Type B HATS |
KAT1 (HAT1) |
Cytoplasm |
H3, H4, H2A |
Histone deposition, DNA repair |
| HAT4 (NAA60) |
Cytoplasm |
H4, H2A |
- |
The Table information is cited from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4881052/ and https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6311713/
2.2 Histone Deacetylases (HDACs)
HDACs, also known as "erasers", are enzymes that remove acetyl groups from acetylated histones. Histone deacetylation reduces DNA accessibility by exposing the positive charge of the lysine residues, which enables interactions between DNA and the histone tail, leading to chromatin compaction and a more condensed chromatin structure, effectively repressing gene expression.
HDACs are vital for various physiological processes, including development and cellular homeostasis, while also contributing to pathological conditions such as neurodegenerative disorders, genetic diseases, and cancer.
In humans, there are four classes of HDAC enzymes based on their sequence homology to yeast [4]. Class I HDACs include HDAC1-3 and HDAC8. Class II HDACs consist of HDAC4-7, HDAC9, and HDAC10 [5-7]. The Class III HDACs, also known as sirtuins, include SIRT1-7 [8,9], which require the NAD+ cofactor for activity. The Class IV HDAC only has an enzyme HDAC11 [10]. Class I, II, and IV HDACs are Zn2+-dependent amidohydrolases.
| Deacetylases Family |
Class |
Subclass |
Members |
Cell Compartment |
Histone Substrate |
Biological relevance |
| Classical (Zn2+-dependent) |
Class I |
|
HDAC1 |
Nucleus |
all four core histones [11] |
Mediates deacetylation of lysine on the N-terminal part of the core histones and plays and important role in transcriptional regulation, cell cycle progression and development event |
| HDAC2
|
Nucleus |
all four core histones [11] |
| HDAC3
|
Nucleus |
H4K5 H4K12 H2AK5 |
| HDAC8
|
Nucleus |
preferentially deacetylates histones H3 and H4 [12] |
| Class II |
Class IIa |
HDAC4
|
Nucleus |
all four core histones [11] |
|
| HDAC5
|
Nucleus |
all four core histones [11] |
|
| HDAC7
|
Nucleus |
|
|
| HDAC9
|
Nucleus |
H3K9
H3K14 H3K18 [13]
|
|
| Class IIb |
HDAC6
|
Cytoplasm |
all four core histones [11] |
|
| HDAC10
|
Cytoplasm |
H3K9
H3K14 H3K18 H3K27 [14] |
Involved in MSH2 deacetylation |
| Class IV |
|
HDAC11
|
Nucleus |
H3K9 H3K14 [15]
H4K16
H4K5
H4K12 [16] |
Plays an important role in transcriptional regulation, cell cycle progression and developmental events
|
| NAD+-dependent |
Class III |
|
SIRT1 |
Nucleus |
H3K9
H3K14
H3K56
H4K16
H1K26 [11] |
Chromatin organization, DNA repair/genome stability, cancer |
| SIRT2
|
Cytoplasm |
H4K16
H3K56 [11] |
Chromatin condensation/mitosis, DNA repair, cancer |
| SIRT3
|
Mitochondria |
H4K16 [11] |
Chromatin silencing, DNA repair, cellular stress |
| SIRT4
|
Mitochondria |
/ |
Regulates the cellular metabolic response to DNA damage |
| SIRT5
|
Mitochondria |
/ |
Regulates the mitochondrial lysine succinylome and metabolic networks |
| SIRT6
|
Nucleus |
H3K9
H3K56 [11] |
Telomeric chromatin/senescence, DNA repair/genome stability, energy metabolism |
| SIRT7
|
Nucleolus |
H3K18 [11] |
Mediates deacetylation of H3K18ac
Mediates deacetylation of lysine residues on the N-terminal part of the core histones |
The Table information is cited from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3970420/ and https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7444376/
HDACs are expressed in various tumors and function differently. Hypoacetylation is a prevalent characteristic observed in numerous cancers. Class I and II HDACs are generally considered oncoproteins that interact with substrates and co-repressors to regulate gene expression, promoting tumorigenesis and cancer development. However, SIRTs (Class III HDACs) can function as both oncoproteins and tumor suppressors, depending on the context.
2.3 Readers
Histone acetylation readers specifically recognize acetylated lysines and recruit transcription factors and other regulatory proteins to bind to the DNA, initiating transcription, thus activating gene expression [17,18]. They are usually bromodomain (BRD)-containing proteins or themselves acetyllysine-binding proteins, such as the BRDs and extra-terminal domain (BET) family, and are responsible for interpreting the acetylation signal into specific changes in gene expression and chromatin remodeling.
BRDs stand out as the sole protein group capable of recognizing and binding acetylated histone lysine residues. They are extensively distributed throughout various tissues and categorized into eight families based on sequence or structural similarity, each displaying diverse activities such as histone modification and chromatin remodeling.
| BRDs |
Members |
| Class I |
BAZ1A, BPTF, CECR2, GCN5L2,
PCAF |
| Class II |
BRD2, BRD3, BRD4, BRDT
|
| Class III |
BAZ1B, BRD8B, BRWD3, CREBBP, EP300, PHIP, WDR9
|
| Class IV |
ATAD2, ATAD2B, BRD1, BRD7, BRD9, BRPF1, BRPF3
|
| Class V |
BAZ2A, BAZ2B, SP100, SP110, SP140, SP140L, TRIM24, TRIM33, TRIM66
|
| Class VI |
MLL, TRIM28
|
| Class VII |
BRWD3, PHIP, TAF1, TAF1L, WDR9, ZMYND8, ZMYND11
|
| Class VIII |
ASH1L, PBRM1, SMARCA2, SMARCA4
|
The Table information is cited from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7686570/
3. Mechanism of Histone Acetylation
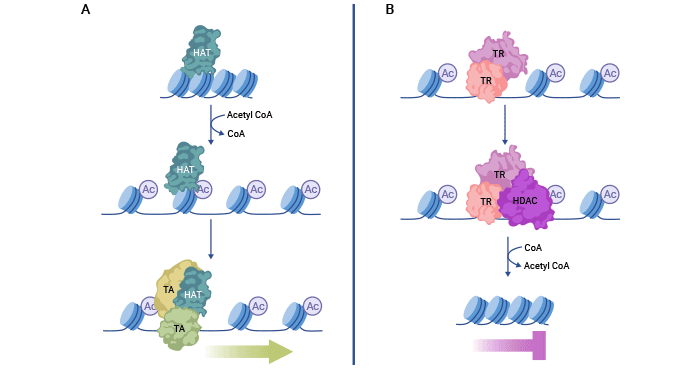
HATs acetylate histone lysine residues, which decreases the electrostatic interactions between histones and DNA, leading to a relaxed chromatin structure. Consequently, DNA becomes exposed, facilitating the recruitment of transcriptional activators (TA) to genomic sequences and activating gene expression.
Conversely, HDACs can interact with transcriptional repressor (TR) complexes to remove these acetyl modifications. This enhances the electrostatic interactions between DNA and histones, leading to compact chromatin that represses transcription. Maintaining a balance between histone acetylation and deacetylation is crucial for proper gene expression.
Figure 1. Histone acethylation and deacetylation mechanism
The picture is cited from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7820808/
4. Function of Histone Acetylation
A large amount of research data demonstrated that histone acetylationis widespread in cells and is involved in various cellular activities, including regulation of nucleosome assembly, folding and deconvolution of chromatin, gene transcription, DNA damage repair, cell cycle, and apoptosis [19].
5. Crosstalk with Other Modifications
There exists intricate crosstalk between histone acetylation and other histone modifications, such as methylation, phosphorylation, and ubiquitination. They cooperate or antagonize each other, influencing chromatin structure and gene expression patterns.
In the budding yeast Saccharomyces cerevisiae, Snf1 kinase-mediated H3S10ph promotes the acetylation of H3K14 by the Gcn5 acetyltransferase, amplifying the interaction between histone H3 and the 14-3-3 proteins Bmh1 and Bmh2 during gene activation [20]. In mammalian cells, H3K18ac and H3K23ac facilitate CARM1 methyltransferase-mediated H3R17 methylation, leading to activation of estrogen-responsive genes [21].
Histone modification crosstalk can also play a role in removing specific modifications. For instance, in budding yeast, the Set2 methyltransferase, which associates with RNA polymerase II, methylates H3K36, directing nucleosomes for deacetylation of both H3 and H4 by the Rpd3S deacetylase complex following RNA polymerase passage [22].
Wojcik, F. et al. demonstrated that acetylation of H2A on multiple sites, including K5, K9, K13, and K15 inhibits the ubiquitylation of H2BK120 [23].
6. Development and Disease
Histone acetylation is involved in numerous biological processes, including development, differentiation, and response to environmental cues. Dysregulation of histone acetylation has been associated with various diseases, such as cancer, neurological disorders, and metabolic disorders, highlighting its significance in disease pathogenesis.
Aberrant histone acetylation patterns have been observed in cancer cells, contributing to uncontrolled cell growth and tumorigenesis. HDAC inhibitors, which restore proper acetylation patterns, have shown promise as potential therapies for certain cancers, offering a novel approach to target cancer cells while sparing normal cells.
In neurodegenerative diseases, such as Alzheimer's and Parkinson's, disturbances in histone acetylation have been linked to changes in gene expression patterns associated with neuronal survival and function. Dysregulated histone acetylation may contribute to neurodegeneration and disease progression.
Moreover, histone acetylation is also associated with metabolic disorders, where it can impact the expression of genes involved in glucose metabolism and lipid homeostasis. Epigenetic changes in response to diet and environmental factors may contribute to metabolic dysregulation and obesity-related diseases.
Together, HATs, HDACs, and BRDs form a complex regulatory network that controls the acetylation status of histones and, consequently, the accessibility of genes for transcription. This epigenetic machinery plays a critical role in various biological processes, including development, differentiation, and response to environmental cues. Dysregulation of these enzymes can lead to aberrant gene expression patterns and is associated with several diseases, making them promising targets for therapeutic interventions and drug development.
References
[1] Allfrey VG, Faulkner R, Mirsky AE. Acetylation and methylation of histones and their possible role in the regulation of RNA synthesis [J]. Proc Natl Acad Sci USA. 64;51:786–794.
[2] Roth, S.Y.; Denu, J.M.; Allis, C.D. Histone acetyltransferases [J]. Annu. Rev. Biochem. 2001, 70, 81–120.
[3] Perez-Campo FM, Costa G, et al. The MYSTerious MOZ, a histone acetyltransferase with a key role in haematopoiesis. Immunology. 2013 Jun;139(2):161-5.
[4] Seto E, Yoshida M. Erasers of histone acetylation: the histone deacetylase enzymes [J]. Cold Spring Harb Perspect Biol. 2014;6(4):a018713–a.
[5] Grozinger CM, Hassig CA, Schreiber SL 1999. Three proteins define a class of human histone deacetylases related to yeast Hda1p [J]. Proc Natl Acad Sci 96: 4868–4873.
[6] Kao HY, Downes M, Ordentlich P, Evans RM 2000. Isolation of a novel histone deacetylase reveals that class I and class II deacetylases promote SMRT-mediated repression [J]. Genes Dev 14: 55–66.
[7] Kao HY, Lee CH, Komarov A, Han CC, Evans RM 2002. Isolation and characterization of mammalian HDAC10, a novel histone deacetylase [J]. J Biol Chem 277: 187–193.
[8] Frye RA 1999. Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity [J]. Biochem Biophys Res Commun 260: 273–279.
[9] Du J, Zhou Y, et al. 2011. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase [J]. Science 334: 806–809.
[10] Gao L, Cueto MA, Asselbergs F, Atadja P 2002. Cloning and functional characterization of HDAC11, a novel member of the human histone deacetylase family [J]. J Biol Chem 277: 25748–25755.
[11] Seto E, Yoshida M. Erasers of histone acetylation: the histone deacetylase enzymes [J]. Cold Spring Harb Perspect Biol. 2014 Apr 1;6(4):a018713.
[12] Hu E, Chen Z, et al. 2000. Cloning and characterization of a novel human class I histone deacetylase that functions as a transcription repressor [J]. J Biol Chem 275: 15254–15264.
[13] Kailin Yan, Qiang Cao, et al.Histone Deacetylase 9 Deficiency Protects against Effector T Cell-mediated Systemic Autoimmunity [J]. Immunology, Volume 286, Issue 33, 19 August 2011, Pages 28833-28843.
[14] Li Y, Peng L, Seto E. Histone Deacetylase 10 Regulates the Cell Cycle G2/M Phase Transition via a Novel Let-7-HMGA2-Cyclin A2 Pathway [J]. Mol Cell Biol. 2015 Oct;35(20):3547-65.
[15] Liu H, Hu Q, D'ercole AJ, Ye P. Histone deacetylase 11 regulates oligodendrocyte-specific gene expression and cell development in OL-1 oligodendroglia cells [J]. Glia. 2009 Jan 1;57(1):1-12.
[16] Huang R, Sui L, et al. HDAC11 inhibition disrupts porcine oocyte meiosis via regulating α-tubulin acetylation and histone modifications [J]. Aging (Albany NY). 2021 Mar 19;13(6):8849-8864.
[17] Jain, A. K., and Barton, M. C. (2017). Bromodomain histone readers and cancer [J]. J. Mol. Biol. 429 (13), 2003–2010.
[18] Dhalluin C, Carlson JE, Zeng L, He C, Aggarwal AK, Zhou MM. Structure and ligand of a histone acetyltransferase bromodomain [J]. Nature. 1999;399:491–496.
[19] Chen H, Tini M, Evans RM. HATs on and beyond chromatin [J]. Curr Opin Cell Biol (2001) 13:218–24.
[20] Wendy Walter, David Clynes, et al. 14-3-3 Interaction with Histone H3 Involves a Dual Modification Pattern of Phosphoacetylation [J]. Mol Cell Biol. 2008 Apr; 28(8): 2840–2849.
[21] Daujat S, Bauer UM, Shah V, et al. Crosstalk between CARM1 methylation and CBP acetylation on histone H3 [J]. Current Biology : CB. 2002 Dec;12(24):2090-2097.
[22] Jung-Shin Lee and Ali Shilatifard. A site to remember: H3K36 methylation a mark for histone deacetylation [J]. Mutat Res. 2007 May 1;618(1-2):130-4.
[23] Wojcik, F., Dann, G.P., Beh, L.Y. et al. Functional crosstalk between histone H2B ubiquitylation and H2A modifications and variants. Nat Commun 9, 1394 (2018).