Histone Methylation
A link between histone methylation and DNA transcription was first described in 1999, but unlike histone acetylation, it was quickly followed by the identification of specific histone methyltransferases (HMTs) [1,2].
1. What Is Histone Methylation?
Histone methylation is the methylation that occurs on lysine (K) or arginine (R) residues at the N-terminal of the H3 and H4 histones. Histone arginine residues can be mono-(me1), asymmetrically or symmetrically di-methylated (me2as or me2s), whereas lysine residues can undergo mono-, di- (me2), and tri-methylation (me3).
There are six extensively studied methylation sites in mammalian cells, including H3K4, H3K9, H3K27, H3K36, H3K79, and H4K20. As for arginine methylation, it predominantly occurs at H3R2, H3R8, H3R17, H3R26, and H4R3 [3]. Unlike histone acetylation, which causes transcriptional activation through weakening histone-DNA interaction, histone methylation increases the basicity and hydrophobicity of histones and affects DNA-transcription factors interaction, activating or inhibiting gene expression.
2. Three Essential Components Involved in Histone Methylation
Histone methylation is accomplished through the action of three essential components, including the writers, readers, and erasers. Writers, also known as histone methyltransferases (HMTs), transfer methyl groups from S-adenosyl-l-methionine (AdoMet) to histone tails. Readers recognize and bind to methyl groups to ultimately influence gene expression. Erasers, also called histone demethylases (HDMs), remove methyl groups. HMTs and HDMs function collaboratively to maintain appropriate global histone methylation levels, thereby governing gene expression patterns.
2.1 Histone Methyltransferases
Histone methyltransferases (HMTs) are divided into two classes according to their structure and modification positions: histone lysine methyltransferases (KMTs) and protein arginine methyltransferases (PRMTs). Different enzymes are responsible for methylation at different sites.
KMTs mono-, di-, or tri-methylate the ε-nitrogen of a histone lysine residue using the S-adenosyl-L-methionine as the methyl group donor. According to the catalytic domain sequence, KMTs are subdivided into two families: SET domain-containing KMTs and non-SET domain-containing KMTs.
PRMTs mono- or di-methylate the guanidyl group of a histone arginine residue. They are mainly classified into type I and type II enzymes and initiate the formation of a mono-methylated (MMA) intermediate. And then, asymmetric dimethylation of arginine residues (aDMA) and symmetric dimethylation of arginine residues (sDMA) are produced by type I PRMTs (PRMT 1-4, 6, 8, and 9) and type II PRMTs (PRMT5, and 7), respectively.
2.2 Histone Demethylases
The first discovery in 2004 of LSD1 as an enzyme that specifically demethylates histone H3K4 was followed by the identification of several demethylases playing regulatory roles in transcription. Demethylases’ discovery corrected that the histone methylation is reversible. There are two major KDM families: the KDM1 family and the Jumonji C (JmjC) domain- containing family.
| Classification |
Histone Demethylases |
Substrate Specificity |
Function |
| KDM1 family |
KDM1A/LSD1 |
H3K4me1/2, H3K9me1/2 |
Transcription activation or repression, heterochromatin formation |
| KDM1B/LSD2 |
H3K4me1/2 |
Transcription repression |
| JmjC family |
KDM2A |
H3K36me1/2 |
Transcription elongation |
| KDM2B |
H3K36me1/2 |
Transcription elongation |
| KDM3A |
H3K9me1/2 |
Androgen receptor gene activation, spermatogenesis |
| KDM3B |
H3K9me1/2 |
Trabscription activation |
| KDM4A |
H3K9me2/3, H3K36me2/3 |
Transcription repression, genome integrity |
| KDM4B |
H3K9me2/3, H3K36me2/3 |
Heterochromatin formation |
| KDM4C |
H3K9me2/3, H3K36me2/3 |
Putative oncogene |
| KDM4D |
H3K9me2/3, H3K36me2/3 |
Transcription activation or repression |
| KDM5A |
H3K4me2/3 |
Retinoblastoma-interacting protein |
| KDM5B |
H3K4me1/2/3 |
Transcription repression |
| KDM5C |
H3K4me2/3 |
X-linked mental retardation |
| KDM5D |
H3K4me2/3 |
Male-specific antigen |
| KDM6A |
H3K27me2/3 |
Transcription activation |
| KDM6B |
H3K27me2/3 |
Transcription activation |
| KDM7A |
H3K9me1/2, H3K27me1/2 |
Transcription activation |
| KDM7B |
H3K9me1/2 |
Transcription activation |
| NO66 |
H3K4me2/3, H3K36me2/3 |
Transcription repression |
2.3 Readers
Histone methylation reader proteins recognize methylated histones by methyl-lysine binding motifs, encompassing plant homeodomain (PHD), chromo, tudor, proline-tryptophan-tryptophan-proline (PWWP), WD40, BAH, ADD, ankyrin repeat, MBT, and zn-CW domains. Furthermore, these proteins can methylated lysine targets by assessing their methylation state and the adjacent amino acid sequence.
Different types of reader domains for histone methylation are categorized by their recognition of distinct methylated sites. PHD fingers are responsible for identifying H3K4 methylation, while chromodomains exhibit specificity for K9 or K27 methylation; these chromodomain sub-families are tailored to each respective site. Additionally, PWWP domains are associated with the recognition of K36 methylation.
| PTMs |
Position |
Domains |
Readers |
| Lysine Methylation |
H3K4 |
Chromo |
CHD1 |
| PHD |
RAG2, ING2, BPTF, TAF3, PHF2, ING4, YNG1, PHF8, BHC80, AIRE |
| Tudor |
JMJD2A, JMJD2C, Sgf29 |
| WD40 |
WDR5, WDR9 |
| ADD |
Dnmt3L |
| MBT |
PHF20L1 |
| Zf-CW |
ZCWPW1 |
| H3K9 |
Chromo |
HP1, CDY1, CDYL, CDYL2 |
| PHD |
SMCX |
| Tudor |
TDRD7, UHRF1 |
| WD40 |
EED, LRWD1 |
| Ankyrin Repeats |
G9a/GLP |
H3K27
|
Chromo |
PC, CDYL, CDYL2, CBX7, MPP8 |
| WD40 |
EED, LRWD1 |
H3K36
|
PWWP |
DNMT3A, BRPF1, NSD1, NSD2, NSD3, MSH-6, N-PAC |
| Chromo |
Eaf3, MSL3, MRG15 |
| H3K79 |
Tudor |
53BP1 |
H4K20
|
Tudor |
53BP1/Crb2, PHF20 |
| MBT |
PHF20L1, L3MBTL1,Sfmbt1 |
| WD40 |
LRWD1 |
| PWWP |
Pdp1 |
| H1K26 |
MBT |
L3MBTL1 |
| WD40 |
EED |
| Arginine Methylation |
H3R17 |
Tudor |
TDRD3 |
| H4R3 |
Tudor |
TDRD3 |
| ADD |
Dnmt3a |
The table information is cited from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3131977/
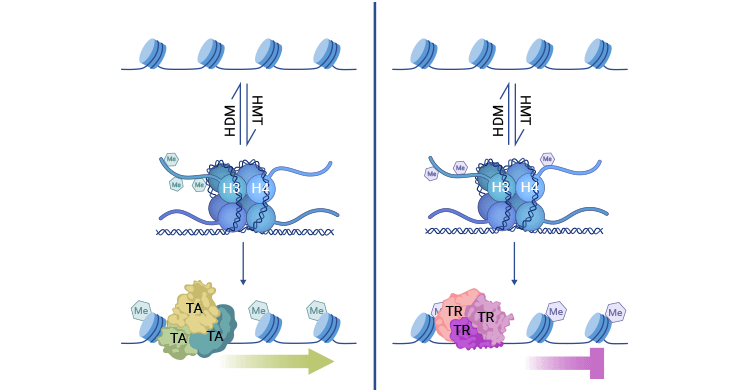
3. Mechanism of Histone Methylation
Histone methylation exerts its control over gene expression by enlisting various transcription factors rather than directly modifying chromatin structure. When HMTs add methyl groups to lysine or arginine residues on H3 and H4 associated with active chromatin, transcriptional activators (TA) are attracted to these sites, promoting gene expression.
On the other hand, when HMTs target different residues on H3 and H4 linked to chromatin repression, the methylated regions can attract transcriptional repressors (TR), leading to gene silencing. The transcriptional impact of histone methylation can be reversed by HDMs. Maintaining proper gene expression relies on the balanced activity of HMT and HDM enzymes.
Figure 1. Histone methylation mechanism
The picture is cited from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7820808/
4. Function of Histone Methyaltion
Histone methylation is a dynamic and reversible process, playing crucial roles in various biological events, including heterochromatin formation, X-chromosome inactivation, genome stability, transcriptional regulation, cell growth, metabolism, signal transduction, and DNA damage response (DDR) [4,5].
Histone methylation can either activate or repress gene expression, which is mainly determined by methylation sites and methylation levels (number of added methyl groups). For example, H3K9me2/3, H3K27me2/3, and H4K20me are involved in gene repression, while H3K27me1, H3K4me2/3, H3K36me3, and H3K79me2/3 are linked to gene activation.
5. The Interplay between Histone Methylation and DNA Methylation
DNA methylation entails the addition of a methyl group to the cytosine base of DNA, predominantly at CpG dinucleotides. DNA methylation patterns are associated with gene silencing and stable transcriptional repression.
There are extensive associations and crosstalk between histone methylation and DNA methylation. DNA methylation at some genomic sites is dependent on histone methylation. The modification status and sequence of DNA can affect the methylation status of histone proteins in chromatin.
Kondo et al. confirmed that H3K9 methylation has a synergistic effect with DNA methylation in gene silencing mechanism, while H3K4 methylation antagonizes gene silencing caused by DNA methylation [6].
David Allis et al. revealed that DNMT3A preferentially selects H3K36 dimethylation regions to promote intergenic DNA methylation, and found that selective loss of intergenic DNA methylation occurred in both blood samples of Sotos syndrome patients and tumors with NSD1 mutations [7]. This discovery has expanded the precise regulatory mechanism between H3K36 methylation and DNA methylation modification, having important biological implications for understanding the occurrence of related human genetic diseases.
6. Histone Methylation and Diseases
The different sites and states of histone methylation can evolve into various patterns of methylation modifications, adding complexity and diversity to gene expression regulated by histone methylation. HMTs and HDMs meticulously maintain the balance between histone methylation and demethylation. Any alterations including mutations in or changed expression of histone methyl-modify enzymes and methyl-binding proteins that disturb this imbalance can lead to various diseases, particularly cancer [8].
For example, the H3K27me3 methyltransferase is upregulated in a number of cancers, including prostate cancer, breast cancer, and lymphomas [9-11]. Histone methyltransferases NSD1 and EZH2 are overexpressed in many tumors, while DOT1L plays a significant role in leukemia. Histone demethylases KDM1A and KDM5B are respectively overexpressed in poorly differentiated neuroblastoma and prostate cancer. The direct interaction between LSD1 and p53 reduces p53 activity, leading to decreased expression of p21 thus inducing tumorigenesis. The abnormal activity of methylation reader proteins is also linked to many human diseases, including developmental disorders and cancer.
Histone methylation is involved in developmental gene expression from pre-fertilization to late birth. Defects in methylation affect various developmental processes and can lead to developmental arrest and lethality in mature animals, or specific defects in organ function, depending on the nature and cell type specificity of the methylation defect.
The deficiency of different regulators of histone methylation has profound consequences on the early stages of embryogenesis and the development of organs. For example, the absence of KDM6B results in the cessation of cardiomyocyte proliferation during late development. The complete deletion of the H3K4me3 demethylase KDM5C leads to neurodevelopmental abnormalities and hinders cortical development in mice.
References
[1] Strahl BD, Ohba R, Cook RG, Allis CD. Methylation of histone H3 at lysine 4 is highly conserved and correlates with transcriptionally active nuclei in Tetrahymena [J]. Proc Natl Acad Sci U S A. 1999;96:14967–14972.
[2] Rea S, Eisenhaber F, O’Carroll D, et al. Regulation of chromatin structure by site-specific histone H3 methyltransferases [J]. Nature. 2000;406:593–599.
[3] Klose, R. J., and Zhang, Y. (2007). Regulation of histone methylation by demethylimination and demethylation [J]. Nat. Rev. Mol. Cell Biol. 8, 307–318.
[4] Martin C, Zhang Y. The diverse functions of histone lysine methylation [J]. Nat Rev Mol Cell Biol 2005;6:838–849.
[5] E.L. Greer, Y. Shi. Histone methylation: A dynamic mark in health, disease and inheritance [J]. Nat. Rev. Genet., 13 (2012), pp. 343-357.
[6] Kondo Y, Shen L. Issa J P. Critical role of histone methylation in tumor suppressor gene silencing in colorectal cancer [J]. Mol Cell Biol. 2003. 23(1); 206~215.
[7] David Allis et al. The histone mark H3K36me2 recruits DNMT3A and shapes the intergenic DNA methylation landscape [J]. Nature. 573: 281-286 (2019).
[8] Chi P, Allis CD, Wang GG. Covalent histone modifications--miswritten, misinterpreted and mis-erased in human cancers [J]. Nature reviews. Cancer. 2010;10:457–69.
[9] Varambally S, et al. The polycomb group protein EZH2 is involved in progression of prostate cancer [J]. Nature. 2002;419:624–9.
[10] Kleer CG, et al. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells [J]. Proc Natl Acad Sci U S A. 2003;100:11606–11.
[11] Visser HP, et al. The Polycomb group protein EZH2 is upregulated in proliferating, cultured human mantle cell lymphoma [J]. British journal of haematology. 2001;112:950–8.