What Are Synaptic Vesicles?
Synaptic vesicles (SVs) are filled with various neurotransmitters and cluster at presynaptic terminals. These vesicles are equipped with two types of obligatory components transport proteins and trafficking proteins. Transport proteins are responsible for neurotransmitter uptake. Trafficking proteins are involved in synaptic vesicle exocytosis, endocytosis, and recycling.
According to their functions and location in the axon terminal, synaptic vesicles are classified into three pools: the readily releasable pool (RRP), the recycling pool, and the reserve pool.
The readily releasable pool, a collection of vesicles that are docked on the presynaptic membrane, is immediately released on stimulation. Vesicles of the RRP are first to undergo fusion upon the invasion of an action potential into the presynaptic terminal. The readily releasable pool is small and is quickly worn out.
The recycling pool, almost close to the cell membrane, refers to a cluster of vesicles that are continuously released under physiological moderate stimulation, the number of which is about three times than RRP vesicles. The vesicles of the recycling pool are used to supplement RRP, so its vesicle number is often in dynamic change. It is also the decisive step for the sustained release of synapses under continuous stimulation.
The reserve pool (RP) contains vesicles that are released under highly intense stimulation. RP is a replenishment of vesicles under high-frequency stimulation.
What Is Synaptic Vesicle Cycle?
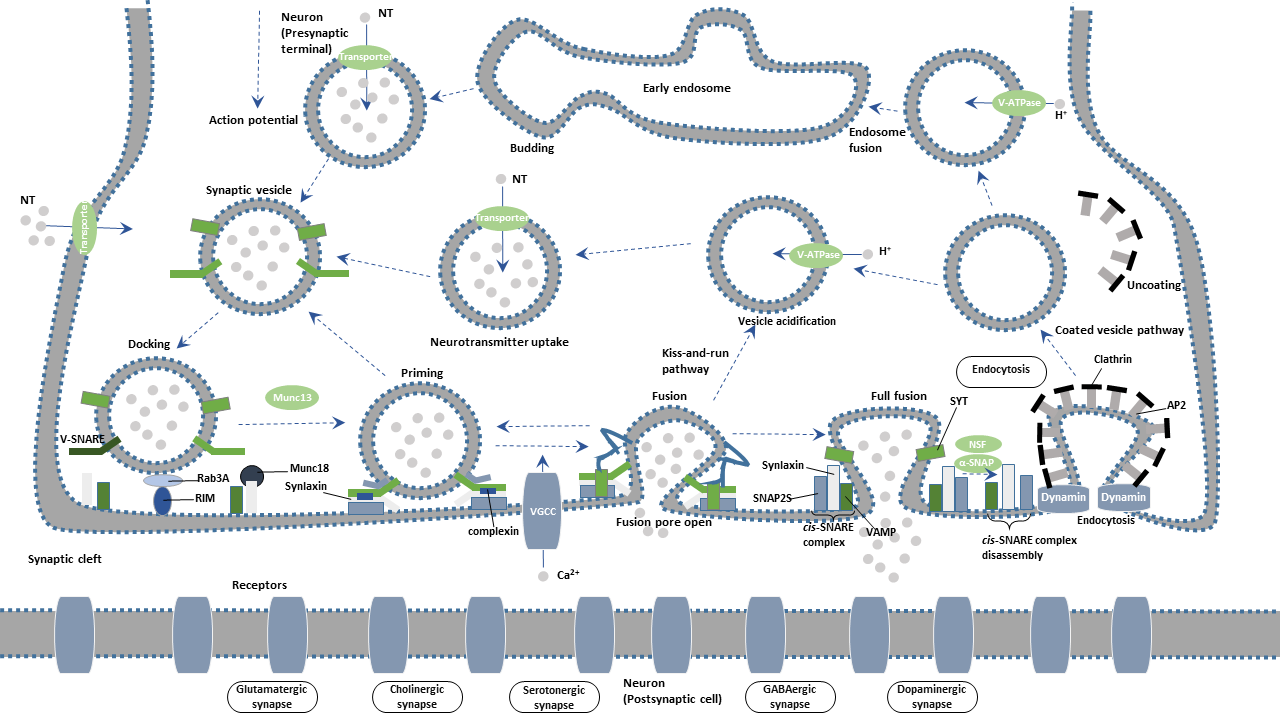
Synaptic vesicles undergo a complex trafficking cycle. Synaptic vesicles undergo a complex trafficking cycle. The whole cycle is divided into six steps, including the formation of synaptic vesicles, the docking of synaptic vesicles in the active zone of the presynaptic membrane, the priming of synaptic vesicles, the fusion of synaptic vesicles with the presynaptic membrane, the release of neurotransmitters by exocytosis, and the endocytosis of vesicles.
The Function of Synaptic Vesicle Cycle
Synaptic vesicles are essential for propagating nerve impulses between neurons. The synaptic vesicle cycle achieves the reuse of synaptic vesicles. Importantly, the cycling provides a sustained replenishment for vesicles containing neurotransmitters to prevent the information from being interrupted due to the loss of synaptic vesicles.
The Mechanism of Synaptic Vesicle Cycle
The synaptic cycle can be divided into the following steps.
The Formation of Synaptic Vesicle:
The proton pump produces proton when ATP is converted into ADP through ATPase activation. The resulting proton gradient provides the energy for the trafficking transmitter into the vesicle. Vesicle transmitter transporter is responsible for transmitter uptake.
Docking:
Synapsin is one of the most important molecules that regulate synaptic vesicle function. In the resting state, Synapsin binds synaptic vesicles to cytoskeleton F-actin, restricting the free movement of vesicles and thus preventing the releasing of vesicle contents. Upon action potential reaching the presynaptic terminal, calcium ions (Ca2+) influx through voltage-gated calcium channel (VGCC) activates calmodulin kinase II (CamK II), mediating the phosphorylation of Synapsin. Phosphorylated Synapsin dissociates and releases synaptic vesicles from the cytoskeleton. Synaptic vesicles are moved to the active zone of the presynaptic plasma membrane. The process is deemed as "docking".
Priming:
When the synaptic vesicles are docked at the active zone, they must be transformed into releasable vesicles. The process is termed as "priming". Studies have been confirmed that RIM (Rab3a interacting molecule), Munc13 and Munc18 are involved in the "priming" of the fusion.
Fusion:
Syntaxin and SNAP-25, interact with Synaptobrevin (also called VAMP or vesicle-associated membrane protein) to promote the final fusion of the synaptic vesicle with the cellular membrane. The vesicle membrane protein synaptotagmin binds to calcium ions, triggering the complete fusion.
Exocytosis:
SNARE (soluble N-ethylmaleimide-sensitive factor attachment receptor), a complex formed by SNAP-25, Syntaxin-1, and Munc-18, is an important player in the synaptic vesicle exocytosis. The vesicle membrane protein synaptotagmin acts as a calcium sensor in the event. Neurotransmitters in the synaptic vesicles are released from a fusion pore at the active site. The released neurotransmitters further bind to the receptor on the postsynaptic membrane, completing the transmission of the action potential.
Endocytosis:
After Ca2+-dependent fusion, the membrane and proteins of the synaptic vesicles found in the plasma membrane are recycled by endocytosis. Synaptic vesicles become re-filled with neurotransmitters and are ready for the next cycle of depolarization and fusion.
Endocytosis of vesicles after fusion with the presynaptic membrane mainly occurs in two ways: the faster kiss-and-run and the slower clathrin-mediated endocytosis.
● Kiss-and-run
In parallel, exocytosed vesicles are recovered at sites away from the active zone and return to the recycling pool for later reuse. An alternative or complementary route of reuse invokes a mode of exocytosis called "kiss-and-run". During kiss-and-run, synaptic vesicles are thought to form a fusion pore, rather than to fully collapse, with the active zone to release their neurotransmitter content. Synaptic vesicles immediately close the pore and are recycled back into the cell.
● clathrin-mediated endocytosis
Synaptotagmin promotes clathrin-mediated endocytosis by binding to the AP-2 complex. Synaptojanin is an important phosphatase, which is localized to the synaptic vesicle by the membrane protein endophilin. It participates in synaptic vesicles endocytosis and cyclic processes by regulating the synthesis of phosphoinositide and dissociating Clathrin's encapsulation from vesicles.
Diseases Associated with Abnormal Synaptic Vesicle Cycle
In Alzheimer's disease (AD) patients, the levels of some functional proteins involved in synaptic vesicle recirculation were found to significantly decrease. Using gene chip technology to analyze the brain tissue of AD patients, it was found that some genes related to synaptic vesicle circulation decreased, while the synaptic genes encoding other functions remained unchanged.





