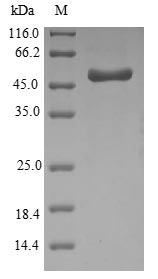
The process of producing recombinant Haemophilus influenzae Outer membrane protein P5 (ompA) starts with synthesizing the target gene, which covers the full length of the mature ompA protein. This gene is fused with an N-terminal 10xHis-SUMO-tag and C-terminal Myc-tag gene and then introduced into an expression vector. The recombinant vector is transformed into E. coli cells, which are induced to express the recombinant ompA protein. These cells are lysed to release the expressed protein. The collected recombinant ompA protein is purified through affinity chromatography. Its purity reaches up to 90% as determined by SDS-PAGE.
Haemophilus influenzae Outer membrane protein P5 (OMPP5) is a significant component of non-typeable Haemophilus influenzae (NTHi) that plays various crucial roles in the pathogenesis and biology of this bacterium. OMPP5 is associated with inorganic polyphosphate and polyhydroxybutyrate, forming large pores in lipid bilayers [1]. It acts as acoli n adhesin essential for the colonization of the chinchilla nasopharynx and infection of the middle ear [2]. The protein exhibits heat modifiability properties similar to major Escherichia outer membrane proteins [3]. OMP P5 is a member of the OmpA family and is a major structural protein in many gram-negative bacteria [4].
Furthermore, OMPP5 has been investigated as a potential immunogen against bacterial otitis media, indicating its importance in vaccine development and immune response [5]. The protein's role as an adhesin that binds to respiratory mucin highlights its significance in NTHi pathogenesis [6].
References:
[1] E. Zakharian and R. Reusch, Haemophilus influenzae outer membrane protein p5 is associated with inorganic polyphosphate and polyhydroxybutyrate, Biophysical Journal, vol. 92, no. 2, p. 588-593, 2007. https://doi.org/10.1529/biophysj.106.095273
[2] J. Bookwalter, J. Jurcisek, S. Gray-Owen, S. Fernández, G. McGillivary, & L. Bakaletz, A carcinoembryonic antigen-related cell adhesion molecule 1 homologue plays a pivotal role in nontypeable haemophilus influenzae
colonization of the chinchilla nasopharynx via the outer membrane protein p5-homologous adhesin, Infection and Immunity, vol. 76, no. 1, p. 48-55, 2008. https://doi.org/10.1128/iai.00980-07
[3] R. Munson, S. Grass, & R. West, Molecular cloning and sequence of the gene for outer membrane protein p5 of haemophilus influenzae, Infection and Immunity, vol. 61, no. 9, p. 4017-4020, 1993. https://doi.org/10.1128/iai.61.9.4017-4020.1993
[4] M. Mullins, K. Register, D. Bayles, C. Loving, T. Nicholson, S. Brockmeieret al., Characterization and comparative analysis of the genes encodinghaemophilus parasuisouter membrane proteins p2 and p5, Journal of Bacteriology, vol. 191, no. 19, p. 5988-6002, 2009. https://doi.org/10.1128/jb.00469-09
[5] J. Kyd, A. Cripps, L. Novotny, & L. Bakaletz, Efficacy of the 26-kilodalton outer membrane protein and two p5 fimbrin-derived immunogens to induce clearance of nontypeable haemophilus influenzae from the rat middle ear and lungs as well as from the chinchilla middle ear and nasopharynx, Infection and Immunity, vol. 71, no. 8, p. 4691-4699, 2003. https://doi.org/10.1128/iai.71.8.4691-4699.2003
[6] R. Hobb, H. Tseng, J. Downes, T. Terry, P. Blackall, M. Takagiet al., Molecular analysis of a haemagglutinin of haemophilus paragallinarum a athe genbank accession numbers for the sequences determined in this work are af491817–af491827., Microbiology, vol. 148, no. 7, p. 2171-2179, 2002. https://doi.org/10.1099/00221287-148-7-2171