What is AMPK Signaling?
AMPK signaling is preserved in tissues from CR animals, maintaining the integrity of the energy metabolism and the efficiency of fat metabolism. AMPK Signaling, a fuel sensor and regulator, promotes ATP-producing and inhibits ATP-consuming pathways in various tissues.
The Function of AMPK Signaling
AMP-activated protein kinase (AMPK) is a central regulator of cellular energy homeostasis, playing critical roles in regulating growth and reprogramming metabolism as well as in cellular processes including autophagy and cell polarity.
The kinase is activated in response to stresses that deplete cellular ATP supplies such as low glucose, hypoxia, ischemia, and heat shock. As a cellular energy sensor responding to low ATP levels, AMPK activation positively regulates signaling pathways that replenish cellular ATP supplies. For example, activation of AMPK enhances both the transcription and translocation of GLUT4, resulting in an increase in insulin-stimulated glucose uptake.
The mechanism of AMPK Signaling
AMPK is a heterotrimeric protein complex that is formed by α, β, and γ subunits. Each of these three subunits take on a specific role in both the stability and activity of AMPK.
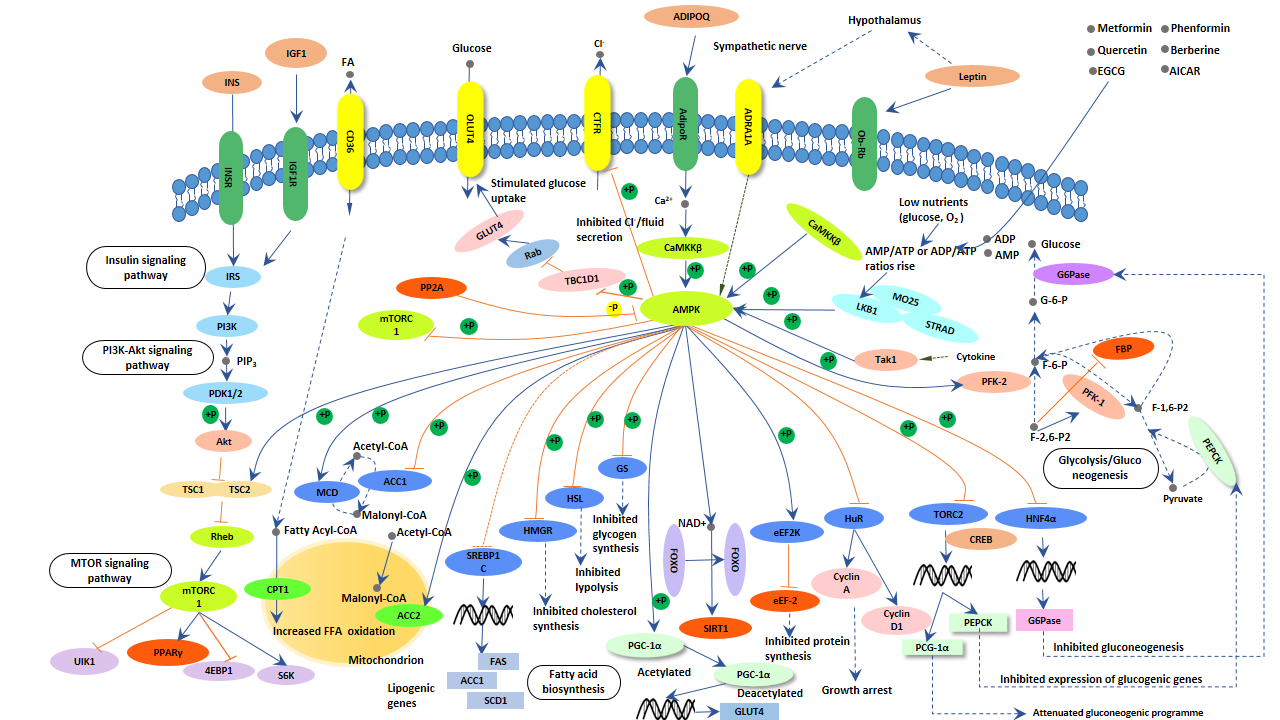
As the figure shows, AMPK activation works via direct phosphorylation of multiple enzymes directly involved in these processes as well as through transcriptional control of metabolism by phosphorylating transcription factors, co-activators, and co-repressors. Because of its role in regulating energy homeostasis, AMPK is considered a potential target in the development of new treatments for obesity, type 2 diabetes, the metabolic syndrome, and cancer.
AMPK Signaling and Related Diseases
AMPK Signaling and Cancer
AMPK was first regarded to link to cancer owing to the discovery that AMPK can mediate the tumor suppressive signaling of liver kinase B1 (LKB1) to the inhibition of mechanistic target of rapamycin complex 1 (mTORC1), which is activated in most cancers. And the LKB1-AMPK axis has been thought as a tumor suppressor pathway through the repression of mTOR1 since the discovery.
In cancer, the role of AMPK exhibts two opposite faces just like a double-edged sword. On one hand, AMPK can protect against cell damage due to oxidative stress, which in turn protects against cancer and tumor initiation. On the other, AMPK promotes energy/glucose uptake by the cells, which can be used by a tumor once it has formed. The activation of AMPK elicits the metabolism reprogramming, which is critical for cell survival during metabolic stress that often occur under pathophysiological conditions such as those observed in the tumor microenvironment.
In a word, AMPK activation is deemed beneficial for the prevention of cancer, but not for its treatment. On the contrary, AMPK inhibition may be helpful for treating established cancers as it can help inhibit the survival and adaptation of tumor stress.
AMPK Signaling and Diabetes
Insulin resistance (IR), dysdunction of beta cell, increased hepatic glucose production are the hallmarks of Type II diabetes, which is a metabolic disease. Once sensing too low cellular energy levels, AMPK is activated and signals to promote glucose uptake in skeletal muscles, fatty acid oxidation in adipose tissues, and decreases hepatic glucose synthesis. Accumulating data has shown that AMPK is out of regulation in animals and humans with Type II diabetes. And AMPK activation renders cells more sensitive to insulin and improves metabolic health.
AMPK Signaling and Neurodegenerative Diseases
Neurodegenerative disorders such as well-known Alzheimer's disease, Parkinson's disease, Huntington's disease, are characterized by nerve cells' progressive degeneration eventually resulting in dementia. These diseases share several features and signaling pathways. In particular, energy metabolism defects, oxidative stress, and excitotoxicity are commonly described and might be associated with AMPK deregulation. AMPK was hyperactive in the brain of patients affected by these neurodegenerative disorders. In response to certain cellular stressors, AMPK activation may exacerbate neuronal atrophy and cell death. AMPK is exactly implicated in the progression of these diseases.





