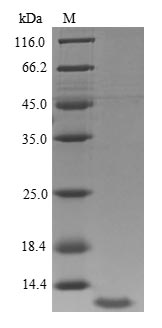
Recombinant Human Insulin-like growth factor I (IGF1) is produced in a yeast expression system and corresponds to the full length of the mature protein, spanning amino acids 49 to 118. This product carries an N-terminal 6xHis tag, which makes purification and detection more straightforward. SDS-PAGE analysis shows a purity level of greater than 90%, which appears suitable for various research applications requiring high-quality protein.
Insulin-like growth factor I (IGF1) represents a crucial protein in cell growth, development, and metabolism. It plays a significant role in regulating growth hormone activity and is integral to various signaling pathways that control cellular proliferation and differentiation. Given these pivotal functions, IGF1 has become extensively studied in fields like endocrinology and oncology, offering valuable insights into growth-related processes and diseases.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
The yeast expression system may support eukaryotic-like folding and disulfide bond formation, which is important for IGF1 (a protein with three disulfide bonds). However, the N-terminal 6xHis tag could potentially interfere with the native structure or function, especially if the tag is near functional domains involved in receptor binding. The purity of >85% indicates the presence of impurities that might include misfolded or inactive proteins. Without experimental validation (e.g., circular dichroism, receptor binding assays, or cell-based bioactivity tests), the folding status and bioactivity remain uncertain. Therefore, while the probability of correct folding is moderate due to the eukaryotic expression system, it is not guaranteed.
1. IGF1 Receptor Binding Studies
This recombinant human IGF1 can be used to investigate binding kinetics and affinity between IGF1 and its cognate receptor (IGF1R) as well as insulin receptor isoform A (IR-A) through surface plasmon resonance or fluorescence polarization assays, but only if the protein is verified to be correctly folded and bioactive. The N-terminal 6xHis tag allows for easy immobilization on nickel-coated surfaces, though it may sterically hinder receptor interactions if the tag affects the binding interface. The purity is >85% (not >90% as originally stated), which could introduce variability due to contaminants. If the protein is misfolded, binding studies may yield inaccurate kinetics and affinity data. It is recommended to perform preliminary bioactivity checks before quantitative assays.
2. Cell Proliferation and Survival Assays
This recombinant IGF1 protein can serve as a positive control or treatment agent in cell culture experiments only if it is properly folded and functional. Researchers can apply it to study IGF1-mediated responses, but the yeast expression system and His tag do not ensure native bioactivity without validation. If misfolded, the protein may fail to stimulate proliferation or survival, leading to false negatives. The 6xHis tag aids purification but might alter biological recognition. Dose-response studies should include controls to confirm activity, and alternative bioactive IGF1 should be used if folding is unverified.
3. Antibody Development and Validation
This purified recombinant IGF1 can work as an immunogen for generating antibodies or as a standard for validation assays, but its effectiveness depends on the folding state. If correctly folded, it can produce antibodies recognizing native IGF1 epitopes; if misfolded, antibodies may target non-native conformations, reducing specificity for physiological IGF1. The His tag provides an additional epitope, but high purity (>85%) still risks antibody cross-reactivity with contaminants. For reliable outcomes, use folding-verified protein or include negative controls.
4. Protein-Protein Interaction Studies
The 6xHis-tagged IGF1 can be used in pull-down assays to identify interacting partners, but interactions may be compromised if the protein is misfolded. The His tag enables immobilization, yet it could mask binding sites or promote non-specific binding. If IGF1 is correctly folded, results can map the interactome; if not, false positives/negatives may occur. The recombinant nature ensures batch consistency, but folding validation is essential for meaningful data.
5. Structural and Biophysical Characterization
This recombinant IGF1 can be applied to structural studies (e.g., crystallography or NMR) only if highly pure and correctly folded. The purity of >85% is suboptimal for high-resolution structural work, typically requiring >95% to avoid heterogeneity. The yeast system may support folding, but the His tag could introduce conformational artifacts. If folded properly, biophysical assays can characterize stability; if misfolded, data will be unreliable. Prior folding checks are critical.
Final Recommendation & Action Plan
Given the uncertainty in protein folding and bioactivity, it is recommended to first validate the recombinant IGF1 using techniques such as circular dichroism to assess secondary structure, ELISA or surface plasmon resonance to confirm receptor binding, and cell-based assays (e.g., proliferation tests on IGF1-responsive cells) to verify bioactivity. If resources allow, compare with a commercially available bioactive IGF1 standard. For immediate use, applications like antibody development may tolerate some folding issues, but critical studies (e.g., receptor binding or structural work) should await validation or use alternative proteins with confirmed activity. Always include appropriate controls in experiments to account for potential folding-related artifacts.