In the 1990s, immunologists made an unexpected discovery: sometimes the most effective way to fight cancer isn't to boost immune responses, but to remove the natural controls that keep them in check.
Think of the immune system like a high-performance car—it has both an accelerator and brakes. For decades, cancer researchers focused mainly on pressing the gas pedal—boosting immune responses to fight tumors. But what if the real breakthrough came from releasing the brakes instead?
This is exactly what happened with the discovery of immune checkpoint inhibitors. These remarkable inhibitors don't just rev up the immune system; they remove the molecular "brakes" that prevent the T cells from attacking cancer cells effectively.
One of the most important of these brakes is a protein called CTLA-4 (Cytotoxic T-Lymphocyte Associated protein 4, also known as CD152). When scientists learned how to block CTLA-4's activity, they opened up entirely new treatment possibilities for patients with advanced cancers like melanoma and lung cancer.
CTLA-4 inhibitors represent a perfect example of how understanding basic biology can lead to therapeutic breakthroughs. By the end of this article, you'll understand exactly how these checkpoint inhibitors work at the molecular level and why this approach has transformed cancer immunotherapy.
Table of Contents
1. What Is CTLA-4's Role in Normal Immune Function?
Before we dive into how CTLA-4 inhibitors work, let's understand what CTLA-4 does under normal circumstances. This protein plays a crucial role as one of the body's primary immune checkpoints—essentially acting as a quality control system that prevents the immune response from going overboard.
1.1 CTLA-4 Structure and Expression
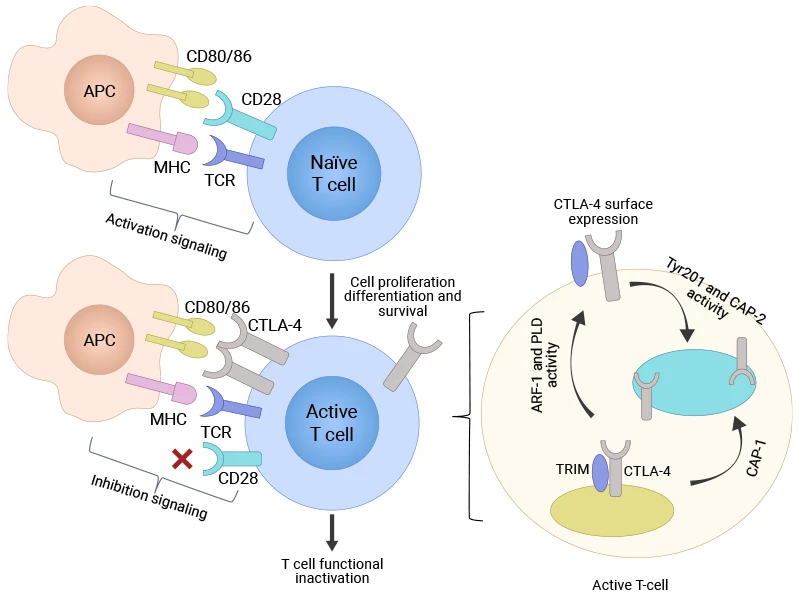
CTLA-4 is a surface protein found in T cells, the immune system's specialized attack cells. The gene that codes for CTLA-4 sits on chromosome 2, and it produces a protein that shares significant structural similarity with another important T cell protein called CD28 [1,2]. This similarity isn't accidental—both proteins compete for the same binding spots on other immune cells.
Here's where it gets interesting. CTLA-4 doesn't show up on all T cells all the time. Instead, it appears mainly on activated T cells that are already responding to a threat. You'll also find high levels of CTLA-4 on regulatory T cells, or "Tregs"—specialized immune cells whose job is to calm down immune responses and prevent autoimmune reactions [3].
1.2 How CTLA-4 Acts as an Immune Brake
To understand how CTLA-4 works, picture a molecular tug-of-war happening on the surface of the T cells. When T cells encounter suspicious cells (like infected or cancerous ones), they need two signals to spring into action. The first signal comes from recognizing foreign proteins. The second comes from CD28 binding to proteins called CD80 and CD86 (key molecules of the B7 family) on antigen-presenting cells (APCs)—this binding delivers an activating "go" signal.
But here's the catch: CTLA-4 binds to these same CD80 and CD86 proteins, and it competes effectively with CD28 for these binding sites [4,5]. When CTLA-4 successfully competes for binding, it delivers the opposite message—a "stop" signal that shuts down T cell activation.
The molecular consequences are dramatic. Instead of activating pathways that promote T cell growth and function—like Ras, PI3K/AKT, and transcription factors NF-κB, AP-1, and NF-AT—CTLA-4 shuts them down [6]. This leads to:
-
Reduced T cell multiplication
-
Decreased production of immune-boosting molecules like interleukin-2
-
Prevention of sustained immune responses
-
Maintenance of self-tolerance (the immune system's ability to recognize healthy cells as "safe")
Figure 1: T-cell activation and CTLA-4 surface expression [21]
This system works brilliantly under normal conditions. CTLA-4 prevents autoimmune diseases by stopping T cells from attacking the body's own tissues. It also helps Tregs maintain immune suppression in areas where inflammation needs to be controlled [7].
The problem arises in cancer. Tumors can exploit this natural brake system, using CTLA-4 to shut down the very immune responses that should be eliminating them. This is where CTLA-4 inhibitors come into play—but we'll explore that mechanism in the next section.
2. How Do CTLA-4 Inhibitors Actually Work?
Now that we understand how CTLA-4 normally keeps immune responses in check, let's explore how scientists learned to disable this molecular brake system. The story of CTLA-4 inhibitors is a perfect example of how basic research can lead to breakthrough treatments.
2.1 Types of CTLA-4 Inhibitors and Their Targets
CTLA-4 inhibitors are specialized antibodies—Y-shaped proteins designed to bind specifically to CTLA-4 and block its function. The first and most well-known of these is ipilimumab, which demonstrated significant survival benefits in clinical trials and received FDA approval for melanoma treatment on March 25, 2011 [8,9]. Another important drug in this class is tremelimumab, though it has had a more complex development path.
These are monoclonal antibodies, meaning they're identical copies of a single antibody type, all targeting the same spot on CTLA-4. Think of them as highly specific molecular locks that fit perfectly onto CTLA-4's key binding sites.
Scientists are now developing next-generation CTLA-4 inhibitors with improved properties. Some have shorter half-lives to reduce side effects, while others are engineered for better selectivity or enhanced tumor penetration [10].
2.2 Molecular Mechanisms of Action
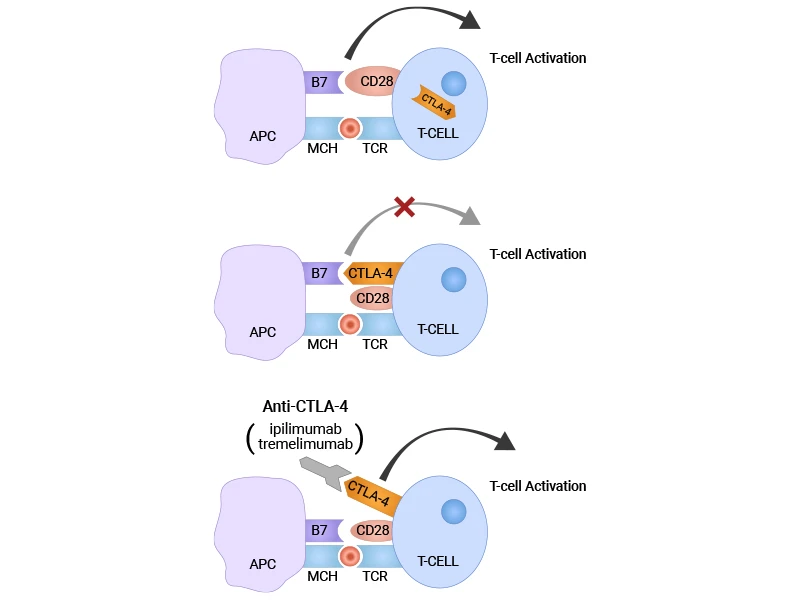
Here's where magic happens. When CTLA-4 inhibitors enter the body, they act like molecular bodyguards for the T cells' "go" signals.
Remember that molecular tug-of-war we discussed? Under normal circumstances, CTLA-4 competes with CD28 for binding to CD80 and CD86 on antigen-presenting cells, sending "stop" signals to T cells. But when CTLA-4 inhibitors are present, they bind directly to CTLA-4 and physically block it from reaching those binding sites [11].
Figure 2: Blocking CTLA-4 with anti-CTLA-4 antibodies (CTLA-4 inhibitors) [22]
With CTLA-4 out of the picture, CD28 can now successfully bind to CD80 and CD86. This restoration of CD28 signaling has cascading effects:
-
Activation of growth-promoting pathways like PI3K/AKT and Ras
-
Increased production of interleukin-2 and other immune-stimulating molecules
-
Enhanced T cell proliferation and clonal expansion
-
Stronger and more sustained anti-tumor immune responses
The result? T cells that were previously held back by CTLA-4's inhibitory signals can now multiply rapidly and launch more effective attacks against cancer cells.
2.3 What Happens to Regulatory T Cells?
One of the most interesting aspects of CTLA-4 inhibition involves what happens to regulatory T cells (Tregs). These cells normally use CTLA-4 to suppress immune responses, particularly in tumor environments where they can protect cancer cells from immune attack.
CTLA-4 inhibitors affect Tregs in two important ways. First, they block CTLA-4's suppressive function, reducing Tregs' ability to calm down other immune cells [12]. Second, and perhaps more importantly, many CTLA-4 inhibitors can actually deplete Tregs through a process called antibody-dependent cellular cytotoxicity (ADCC).
This happens because CTLA-4 is highly expressed on Tregs. When antibodies bind to CTLA-4 on these cells, they can recruit other immune cells like natural killer (NK) cells to destroy the Treg. The specific antibody subclass matters here—some are better at triggering ADCC than others [13].
The end result is a double victory: fewer suppressive Tregs in the tumor microenvironment and more activated effector T cells ready to fight cancer.
This combination can reverse the immune tolerance that many tumors create around themselves, allowing the body's natural defenses to finally recognize and eliminate malignant cells.
Understanding these mechanisms helps explain both why CTLA-4 inhibitors can be so effective against certain cancers and why they sometimes cause autoimmune-like side effects—topics we'll explore in upcoming sections.
3. Where Are CTLA-4 Inhibitors Used Clinically?
With a solid understanding of how CTLA-4 inhibitors work at the molecular level, let's examine where these breakthrough drugs are making the biggest impact in real-world patient care.
3.1 Current FDA-Approved Applications
CTLA-4 inhibitors have proven most successful in treating cancers that are traditionally difficult to manage with conventional therapies. Melanoma was the first major success story—ipilimumab became the first drug to show a survival benefit in patients with advanced melanoma, leading to FDA approval in 2011 [9].
-
Renal cell carcinoma: Ipilimumab combined with nivolumab (a PD-1 inhibitor) is now a first-line treatment for intermediate and poor-risk patients
-
Non-small cell lung cancer: Combination therapy with PD-1/PD-L1 inhibitors has shown remarkable results in certain patient populations
-
Mesothelioma: Recent approvals have given hope to patients with this aggressive cancer linked to asbestos exposure
-
Colorectal cancer: Specifically for tumors with microsatellite instability (MSI-high), which tend to respond better to immunotherapy
The real game-changer has been combination therapies. Rather than using CTLA-4 inhibitors alone, oncologists often pair them with PD-1 or PD-L1 inhibitors. This dual checkpoint blockade approach attacks cancer from multiple angles, often producing response rates that far exceed what either drug achieves individually [14].
3.2 What Determines Clinical Success?
Not all patients respond equally well to CTLA-4 inhibitors, and scientists are working hard to understand why. Several factors seem to influence treatment success.
Tumor immunogenicity—essentially how "foreign" a tumor looks to the immune system—plays a crucial role. Cancers with high mutation loads, like melanoma and lung cancer from smoking, tend to respond better because they present more targets for T cells to recognize [15].
The existing T cell repertoire also matters significantly. Patients need a diverse population of T cells capable of recognizing tumor antigens. Age, previous treatments, and overall immune health all influence this factor.
Interestingly, the tumor microenvironment itself can predict success. Tumors surrounded by immune cells (called "hot" tumors) generally respond better than those in immune-poor environments ("cold" tumors). This has led researchers to explore ways to convert cold tumors into hot ones before starting CTLA-4 inhibitor therapy.
Understanding these factors is helping doctors select the right patients for treatment and develop strategies to improve response rates in those who might not initially be good candidates.
4. What Are the Safety Concerns with CTLA-4 Inhibitors?
While CTLA-4 inhibitors have revolutionized cancer treatment, they come with a unique set of side effects that doctors and patients need to understand. These aren't the typical chemotherapy side effects—instead, they stem directly from the drug's mechanism of action.
Remember how CTLA-4 normally acts as a brake to prevent the immune system from attacking healthy tissues? When we remove those brakes with inhibitors, we sometimes remove too much control. The result can be immune-related adverse events, or "irAEs"—essentially autoimmune reactions where the supercharged immune system starts attacking normal organs.
The most common problems affect skin, digestive system, liver, and hormone-producing glands. Patients might develop severe rashes, diarrhea, or hepatitis that looks very similar to autoimmune diseases [16]. Endocrine issues are particularly tricky—the immune system might attack thyroid, adrenal glands, or pituitary glands, leading to hormone deficiencies that can be permanent.
These side effects can be serious. Studies show that severe irAEs occur in about 10-15% of patients taking ipilimumab alone, and rates can be higher with combination therapies [17,18]. The timing is unpredictable too—some effects appear within weeks, while others might not show up for months.
The good news? Most irAEs are manageable when caught early. Doctors use corticosteroids to calm down the overactive immune response, and they've developed detailed monitoring protocols to catch problems before they become dangerous. Some medical centers even have specialized teams dedicated to managing these unique side effects.
5. What's Next for CTLA-4 Inhibitor Development?
The success of first-generation CTLA-4 inhibitors has sparked an exciting wave of innovation aimed at making these drugs even better. Scientists are approaching this challenge from multiple angles, each addressing different limitations of current treatments.
Next-generation antibody engineering represents one of the most promising frontiers. Researchers are designing CTLA-4 inhibitors with improved selectivity—meaning they can target cancer-fighting T cells more precisely while leaving helpful regulatory functions intact in healthy tissues [19]. Some experimental drugs have shorter half-lives, which could reduce the duration and severity of side effects we discussed earlier.
Other engineering approaches focus on enhanced tumor penetration. Current antibodies sometimes struggle to reach cancer cells buried deep within solid tumors, so scientists are developing smaller, more mobile versions that can navigate these challenging environments more effectively.
The combination therapy revolution is just getting started. While pairing CTLA-4 inhibitors with PD-1 or PD-L1 blockers has already shown remarkable results, researchers are now exploring triple combinations and beyond. Some trials are testing CTLA-4 inhibitors alongside targeted therapies, radiation, or even cancer vaccines [20].
Perhaps most importantly, scientists are racing to develop biomarkers—biological signatures that can predict which patients will benefit most from treatment. This personalized approach could help doctors select the right therapy for each individual while avoiding unnecessary side effects in patients unlikely to respond.
Conclusion
CTLA-4 inhibitors have fundamentally changed how we think about cancer treatment by harnessing the body's own immune system rather than relying solely on toxic chemotherapy agents. By blocking this crucial immune checkpoint, these drugs release the natural brakes that prevent T cells from attacking tumors effectively.
The journey from understanding CTLA-4's basic biology to developing life-saving treatments illustrates how fundamental research can lead to breakthrough therapies. What started as curiosity about immune regulation has evolved into a treatment approach that's giving hope to patients with previously untreatable cancers.
6. Research Tools That Support CTLA-4 Research
Every breakthrough in CTLA-4 research started with solid experimental work. But getting good results depends on having the right reagents.
Many researchers know the frustration of inconsistent results from unreliable products. That's why we focus on creating research tools that work consistently, experiment after experiment.
Our CTLA-4 research portfolio covers the essentials: high-quality proteins, validated antibodies, and sensitive ELISA kits.
Recombinant Proteins
|
Code
|
Product Name
|
Molecule
|
Source
|
Expression Region
|
Tag Info
|
|
CSB-AP005121GU
|
Recombinant Guinea pig Cytotoxic T-lymphocyte associated protein 4 (CTLA4), partial (Active)
|
CTLA-4
|
Yeast
|
37-161aa
|
C-terminal 6xHis-tagged
|
|
CSB-CF006163HU
|
Recombinant Human Cytotoxic T-lymphocyte protein 4 (CTLA4)
|
CTLA-4
|
in vitro E.coli expression system
|
36-223
|
N-terminal 10xHis-tagged
|
|
CSB-MP006163HU2
|
Recombinant Human Cytotoxic T-lymphocyte protein 4 (CTLA4), partial
|
CTLA-4
|
Mammalian cell
|
36-161aa
|
N-terminal Twin-Strep-tagged
|
|
CSB-YP006163HU2
|
Recombinant Human Cytotoxic T-lymphocyte protein 4 (CTLA4), partial
|
CTLA-4
|
Yeast
|
36-161aa
|
N-terminal Twin-Strep-tagged
|
|
CSB-MP006163HU2l4
|
Recombinant Human Cytotoxic T-lymphocyte protein 4 (CTLA4), partial
|
CTLA-4
|
Mammalian cell
|
36-161aa
|
N-terminal Twin-Strep-tagged and C-terminal 6xHis-Avi-tagged
|
|
CSB-EP006163HU1
|
Recombinant Human Cytotoxic T-lymphocyte protein 4 (CTLA4), partial
|
CTLA-4
|
E.coli
|
37-162aa
|
N-terminal 6xHis-tagged
|
|
CSB-MP006163HU2d7
|
Recombinant Human Cytotoxic T-lymphocyte protein 4 (CTLA4), partial
|
CTLA-4
|
Mammalian cell
|
36-161aa
|
C-terminal 10xHis-tagged
|
|
CSB-MP006163HU1
|
Recombinant Human Cytotoxic T-lymphocyte protein 4 (CTLA4), partial (Active)
|
CTLA-4
|
Mammalian cell
|
37-162aa
|
C-terminal hFc-tagged
|
|
CSB-AP005231HU
|
Recombinant Human Cytotoxic T-lymphocyte protein 4 (CTLA4), partial (Active)
|
CTLA-4
|
Mammalian cell
|
36-161aa
|
C-terminal 6xHis-tagged
|
|
CSB-MP006163HU1j1-B
|
Recombinant Human Cytotoxic T-lymphocyte protein 4 (CTLA4), partial,Biotinylated
|
CTLA-4
|
Mammalian cell
|
37-162aa
|
C-terminal mFc-Avi-tagged
|
|
CSB-CF006163MO
|
Recombinant Mouse Cytotoxic T-lymphocyte protein 4 (Ctla4)
|
CTLA-4
|
in vitro E.coli expression system
|
36-223
|
10xHis-SUMO-tag
|
|
CSB-AP005301MO
|
Recombinant Mouse Cytotoxic T-lymphocyte protein 4 (Ctla4), partial (Active)
|
CTLA-4
|
Mammalian cell
|
37-161aa
|
C-terminal 6xHis-tagged
|
|
CSB-EP006163MO1
|
Recombinant Mouse Cytotoxic T-lymphocyte protein 4 (Ctla4),Partial
|
CTLA-4
|
E.coli
|
36-161aa
|
N-terminal 10xHis-SUMO-tagged and C-terminal Myc-tagged
|
|
CSB-CF006163RB
|
Recombinant Rabbit Cytotoxic T-lymphocyte protein 4 (CTLA4)
|
CTLA-4
|
in vitro E.coli expression system
|
36-223
|
N-terminal 10xHis-tagged
|
|
CSB-CF004913CA
|
Recombinant Cat T-cell-specific surface glycoprotein CD28 (CD28)
|
CD28
|
in vitro E.coli expression system
|
21-221
|
N-terminal 10xHis-tagged
|
|
CSB-CF004913CH
|
Recombinant Chicken T-cell-specific surface glycoprotein CD28 homolog (CD28)
|
CD28
|
in vitro E.coli expression system
|
14-221
|
N-terminal 10xHis-tagged
|
|
CSB-CF004913HU
|
Recombinant Human T-cell-specific surface glycoprotein CD28 (CD28)
|
CD28
|
in vitro E.coli expression system
|
19-220
|
N-terminal 10xHis-tagged
|
|
CSB-MP004913HU1
|
Recombinant Human T-cell-specific surface glycoprotein CD28 (CD28), partial
|
CD28
|
Mammalian cell
|
19-152aa
|
C-terminal hFc-tagged
|
|
CSB-CF004913MO
|
Recombinant Mouse T-cell-specific surface glycoprotein CD28 (Cd28)
|
CD28
|
in vitro E.coli expression system
|
20-218
|
N-terminal 10xHis-tagged
|
|
CSB-CF004913RB
|
Recombinant Rabbit T-cell-specific surface glycoprotein CD28 (CD28)
|
CD28
|
in vitro E.coli expression system
|
20-221
|
N-terminal 10xHis-tagged
|
|
CSB-CF004913RA
|
Recombinant Rat T-cell-specific surface glycoprotein CD28 (Cd28)
|
CD28
|
in vitro E.coli expression system
|
20-218
|
N-terminal 10xHis-tagged
|
Antibodies
|
Code
|
Product Name
|
Molecule
|
Species Reactivity
|
Tested Applications
|
|
CSB-PA006163KA01HU
|
CTLA4 Antibody
|
CTLA-4
|
Human
|
ELISA,WB,IHC
|
|
CSB-PA224600
|
CTLA4 Antibody
|
CTLA-4
|
Human,Mouse,Rat
|
ELISA,IHC
|
|
CSB-PA006163LA01HU
|
CTLA4 Antibody
|
CTLA-4
|
Human
|
ELISA, IF
|
|
CSB-PA252417
|
CTLA4 Antibody
|
CTLA-4
|
Human
|
IHC, ELISA
|
|
CSB-PA006163LB01HU
|
CTLA4 Antibody, HRP conjugated
|
CTLA-4
|
Human
|
ELISA
|
|
CSB-MA006163A0m
|
CTLA4 Monoclonal Antibody
|
CTLA-4
|
Human
|
ELISA, WB, IHC
|
|
CSB-RA006163MA1HU
|
CTLA4 Recombinant Monoclonal Antibody
|
CTLA-4
|
Human
|
ELISA, WB, IF, FC
|
|
CSB-RA213310A0HU
|
CTLA4 Recombinant Monoclonal Antibody
|
CTLA-4
|
Human
|
ELISA, IHC
|
|
CSB-RA006163MA2HU
|
CTLA4 Recombinant Monoclonal Antibody
|
CTLA-4
|
Peptostreptococcus magnus
|
ELISA
|
|
CSB-PA005199
|
CD28 Antibody
|
CD28
|
Human
|
WB, ELISA
|
|
CSB-PA070302
|
Phospho-CD28 (Y218) Antibody
|
CD28
|
Human,Mouse
|
WB, ELISA
|
ELISA Kits
References
[1] Brunet, J.F., et al. A new member of the immunoglobulin superfamily--CTLA-4. Nature. 1987;328(6127):267-70.
[2] Harper, K., et al. CTLA-4 and CD28 activated lymphocyte molecules are closely related in both mouse and human as to sequence, message expression, gene structure, and chromosomal location. J Immunol. 1991;147(3):1037-44.
[3] Wing, K., et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322(5899):271-5.
[4] Linsley, P.S., et al. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174(3):561-9.
[5] van der Merwe, P.A., et al. CD80 (B7-1) binds both CD28 and CTLA-4 with a low affinity and very fast kinetics. J Exp Med. 1997;185(3):393-403.
[6] Krummel, M.F., Allison, J.P. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182(2):459-65.
[7] Takahashi, T., et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192(2):303-10.
[8] Hodi, F.S., et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-23.
[9] U.S. Food and Drug Administration. FDA approves ipilimumab for metastatic melanoma. March 25, 2011. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ipilimumab-metastatic-melanoma
[10] Sharma, A., et al. Next-generation anti-CTLA-4 antibodies and prospects for clinical development. Expert Opin Biol Ther. 2021;21(10):1383-1393.
[11] Leach, D.R., et al. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734-6.
[12] Peggs, K.S., et al. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206(8):1717-25.
[13] Simpson, T.R., et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210(9):1695-710.
[14] Larkin, J., et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23-34.
[15] Rizvi, N.A., et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124-8.
[16] Postow, M.A., et al. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158-168.
[17] Weber, J.S., et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol. 2013;31(34):4311-8.
[18] Wang, D.Y., et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4(12):1721-1728.
[19] Wei, S.C., et al. Distinct cellular mechanisms underlie anti-CTLA-4 and anti-PD-1 checkpoint blockade. Cell. 2017;170(6):1120-33.
[20] Larkin, J., et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535-1546.
[21] Hossen, M.M., et al. (2023). Current understanding of CTLA-4: from mechanism to autoimmune diseases. Frontiers in Immunology, 14, 1198365.
[22] Wojtukiewicz, M.Z., et al. (2021). Inhibitors of immune checkpoints—PD-1, PD-L1, CTLA-4: new opportunities for cancer patients and a new challenge for internists and general practitioners. Cancer Metastasis Reviews, 40(3), 949–982.
CUSABIO team. How Do CTLA-4 Inhibitors Work? A Complete Guide for Early-Career Researchers. https://www.cusabio.com/c-21234.html




Comments
Leave a Comment