What Are Adherens Junctions?
Adherens junctions are multiprotein complexes in the epithelial cells that are close to the apical membrane. And they primarily mediate adhesion between homologous cells in all types of tissues. Adherens junctions attach the actin cytoskeleton to the plasma membrane, forming an adhesive contact among epithelial cells or between epithelial cells and the extracellular matrix.
The Classifications of Adherens Junctions
According to the connected parts, adherens junctions can be divided into adhesion belts and focal adhesions.
Adhesion belt, also known as the intermediate junction, refers to the formation of a continuous band-like structure between adjacent cells. Adhesion belt connects two cells by the interaction between actin and cadherin. And it mainly regulates the shape of the cells.
Focal adhesion, also called mechanical attachment, mediates the connection between the cell to the extracellular matrix. It forms through the interaction between actin and integrin. Focal adhesion promotes cell spreading and migration. And it is also involved in cell signaling.
The Function of Adherens Junction
Adherens junctions keep the integrity of the tissues structure. They initiate and stabilize cell adhesion and regulate the actin cytoskeleton. Adherens junctions promote adhesion between homologous cells and facilitate further organization and separation of the tissues during embryonic development. In adults, adherens junctions participate in maintaining tissue homeostasis and controlling epithelial & endothelial cell permeation. Adherens junctions also promote intercellular communication and signaling conduction, mediate contact inhibition of cell growth, increase resistance to apoptosis, and regulate cell shape & polarity.
The Mechanism of Adherens Junction
The Mechanism of Adhesion Belt
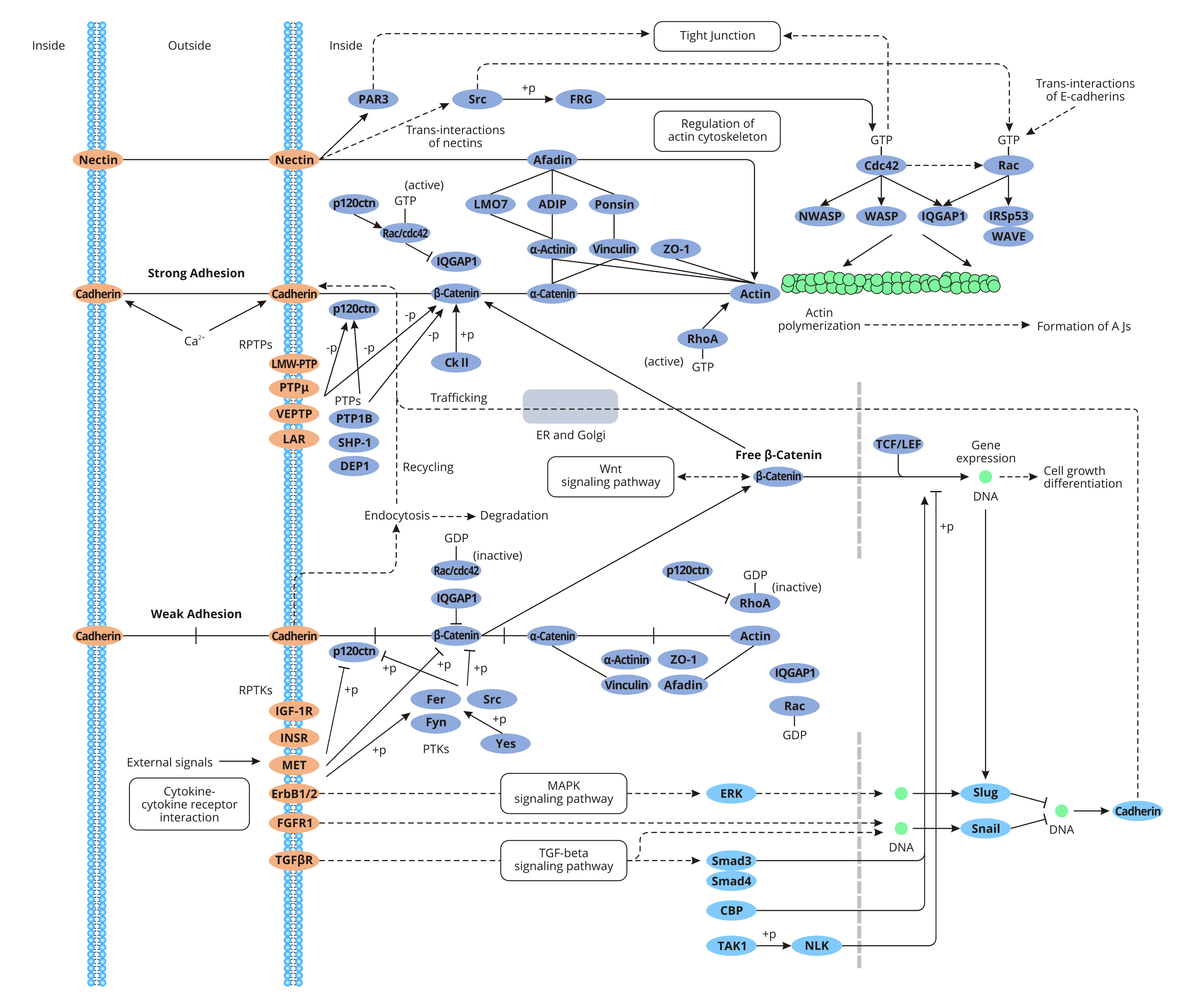
Cadherin is a single-channel transmembrane glycoprotein that belongs to the calcium-dependent adhesion protein of the classical cadherin family. E-cadherin, the main component of the adhesion Belt, is ubiquitous in various epithelial tissues. And it is the main calmodulin (CaM) that keeps epithelial cells adhere to each other. E-cadherin consists of an extracellular domain (5 cadherin repeats: EC1~EC5), a transmembrane domain, and two cytoplasmic domains (the juxtamembrane domain that binds to p120-catenin and the cadherin domain that binds to β-catenin). The EC domain forms a trans-cadherin interaction between adjacent cells, initiates weak cell-cell adhesion, and forms adhesion molecule linkages. The Ca2+ binding in the EC domain ensures the correct conformation of the extracellular domain of cadherin. The cytoplasmic domain includes a highly phosphorylated region that mediates the binding of E-cadherin to β-catenin and participates in signal transduction.
When the highly-conserved cytosolic domain of E-cadherin binds to the intracellular ligand β-catenin, β-catenin is linked to actin to maintain cell polarity, intercellular connectivity, and signal transduction. β-catenin plays a dual role in cells. As a cytoskeletal protein, β-catenin anchors the E-cadherin on the surface of the cell membrane to the cytoskeleton through α-catenin, forming an adhesive junction E-cadherin/β-catenin complex. The E-cadherin/β-catenin complex is the core for the formation of the adhesion belt, which mediates homologous adhesion, maintaining the polarity and integrity of normal epithelium. The adhesion is closely related to tumor invasion and metastasis. As a signal transduction protein, β-catenin takes part in the Wg/Wnt signaling pathway mediated by colon adenomatous polyp protein (APC)/β-catenin/T cytokine/lymphocyte proliferation factor 1 (Tcf1/Lef-1) complex. The pathway is associated with the transformation of epithelial-mesenchymal cells and excessive proliferation and invasion of tumor cells. Down-regulation or deletion of E-cadherin is significantly related to tumor dedifferentiation, intimate growth, metastasis, and poor prognosis.
The Mechanism of Focal Adhesion
Integrins are a class of heterologous cell adhesion molecules that are ubiquitous on the surface of vertebrate cells. And they rely on Ca2+ or Mg2+. Integrins are the core proteins of focal adhesions, mediating the mutual recognition and adhesion between cells and extracellular matrices. So integrins serve as a messenger for the connection between external cells and the internal structure of cells.
Integrins are a transmembrane heterodimer. Two subunits of integrins, alpha and beta chains, are glycosylated and joined together by non-covalent bonds. Integrins bind to extracellular matrix proteins through their extracellular globular domains and link to actin through the intracellular tails, mediating the adhesion of cells to the extracellular matrix.
Integrins are also involved in signal transduction, including both "from the inside to the outside" and "from the outside to the inside" signals. The signal transduction function of integrins is dependent on focal adhesion kinase (FAK), which in turn depends on the formation of adhesion plaques. FAK phosphorylates some proteins, causing a cascade of signal amplification reactions. Signal transduction involving integrins and some other cell surface molecules affects many behaviors of cells, including motion, growth, and cell survival.
Adhesive Junctions and Diseases
Adherens junctions between vascular endothelial cells (EC) is an important basis for angiogenesis and maintenance. Adhesive connections are widely distributed between ECs and are essential for maintaining vascular integrity and stability. It is well known that the integrity of the vessel wall and sustained homeostasis are fundamental to ensure normal blood circulation. If pathological factors in the internal environment such as inflammation and oxygen free radicals, act on vascular endothelial cells, the adhesion between endothelial cells is reduced, which leads to the destruction of vascular integrity and stability, causing vascular endothelial damage and a series of pathological changes such as increased vascular permeability, vascular leakage, and atherosclerosis, leading to cardiovascular and cerebrovascular diseases.
Cancer is a killer that seriously harms human health. In biological behavior, infiltration and metastasis are the most important characteristics of cancer and are the leading cause of death in patients. The loss of cancer cell adhesion is the first and a key step in cancer infiltration and metastasis. The process of tumor invasion and metastasis is an alternating process of adhesion and de-adhesion. The cell connection between the tumor cells and the surrounding environment is dynamically changing. So the strength of the superiority (the balance between adhesion and de-adhesion) determines the behavior of the tumor cells.
Many studies have confirmed that E-cadherin is negatively correlated with tumor invasion and metastasis, probably because E-cadherin can promote the homologous adhesion of tumor cells and keep them shedding from the tumor. In vitro experiments showed that the tumor cell line with E-cadherin expression had no innateness, while the tumor cell line with affinitive phenotype had no E-cadherin expression.





