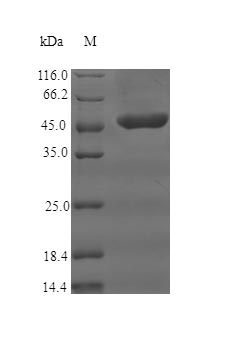
This Recombinant Human Complement receptor type 1 (CR1) is produced using an E.coli expression system, covering amino acids 41 to 234. The protein appears to be partially expressed and includes an N-terminal GST tag, which makes purification and detection more straightforward. Purity levels exceed 90%, as confirmed by SDS-PAGE analysis, suggesting it should work reliably in experimental settings. Endotoxin levels remain minimal, making it appropriate for research applications.
Complement receptor type 1 (CR1) seems to play a crucial role in immune system function by helping clear immune complexes and regulating complement activation. The receptor is involved in both classical and alternative complement pathways, likely protecting host tissues from damage during immune responses. CR1 has drawn considerable attention in studies of various immune-related conditions, which may explain why it's become such a valuable target in immunological research.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Based on the provided information, the recombinant Human CR1 fragment is expressed in E. coli, a prokaryotic system that is generally unsuitable for producing functional eukaryotic extracellular domain proteins. CR1 is a complex transmembrane protein requiring proper folding, glycosylation, and domain organization for its complement-binding functions. The expressed fragment (41-234aa) is partial and lacks the full extracellular domain structure, while the large N-terminal GST tag (∼26kDa) may significantly interfere with proper folding of the CR1 domain. E. coli cannot provide the necessary post-translational modifications (e.g., glycosylation) required for CR1's native conformation. Since activity is explicitly unverified, the protein is highly likely to be misfolded and inactive for its natural complement-binding functions.
1. GST Pull-Down Assays for Protein-Protein Interaction Studies
The N-terminal GST tag technically enables pull-down experiments through glutathione-sepharose binding. However, if the CR1 fragment is misfolded (as expected in E. coli), it will not display native conformational epitopes needed for physiological interactions with complement components or signaling partners. Identified interactions may be non-specific or artifactual. This application should not be used for biological discovery without prior validation of proper folding and activity.
2. Antibody Development and Epitope Mapping
This application is appropriate with caveats. The recombinant CR1 fragment can serve as an immunogen for generating antibodies that recognize linear epitopes within the 41-234aa region. The high purity (>90%) supports immunization protocols. However, if the protein is misfolded, antibodies may not recognize conformational epitopes of native, fully-glycosylated CR1 on cell surfaces. Antibodies should be validated against native CR1 from human cells or tissues.
3. Biochemical Characterization and Biophysical Analysis
This application is well-suited and should be prioritized. Techniques like circular dichroism spectroscopy, size exclusion chromatography, and dynamic light scattering can directly assess the protein's folding state, oligomerization, and stability. These studies are valuable even if the protein is inactive, as they characterize the recombinant product itself. The GST tag may influence the results but can be accounted for in analyses.
4. GST-Tagged Protein Purification Method Development
This application is technically feasible. The GST-CR1 fusion protein can be used as a model system for optimizing E. coli expression and purification protocols for difficult-to-express extracellular domain fragments. However, methods developed using this potentially misfolded protein may not translate well to producing functional CR1 domains. The application has limited biological relevance but can be useful for technical methodology development.
Final Recommendation & Action Plan
Given the high probability of misfolding due to E. coli expression and the large GST tag, this CR1 fragment is primarily suitable for antibody development (with validation against native protein) and biochemical characterization studies. Avoid biological interaction studies (pull-down assays) until proper folding is confirmed. Recommended first steps: 1) Perform biophysical characterization (size exclusion chromatography, circular dichroism) to assess folding state; 2) If possible, test binding to known CR1 ligands (e.g., C3b/C4b) with positive controls; 3) For antibody production, use the protein as immunogen but validate resulting antibodies against native CR1; 4) Consider alternative expression systems (e.g., mammalian cells) for producing functional CR1 domains. The current protein may serve as a negative control in studies requiring properly folded CR1.