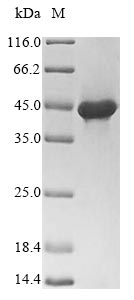
The Glycoprotein precursor (GPC) is a crucial protein found in arenaviruses that undergoes posttranslational cleavage to produce the components forming the GP complex. The GPC is expressed as a polyprotein that is co-translationally cleaved to yield the stable signal peptide (SSP), GP1, and GP2 subunits [1][2]. Maturation of the GPC involves proteolytic cleavage into the SSP, GP1 attachment glycoprotein, and GP2 fusion glycoprotein [3]. The mature GPC complex is metastable and is primed to mediate membrane fusion in response to acidic pH [4]. The virion form of GPC is a trimer of heterodimers containing the receptor-binding subunit GP1 and the fusion-mediating subunit GP2 [5]. The GPC precursor is proteolytically cleaved by the cellular subtilase SKI-1/S1P to yield the mature glycoproteins G1 and G2 [6].
The GPC is essential for the assembly and function of arenaviruses, playing a critical role in viral entry into host cells and serving as a target for antibody-mediated neutralization [5]. The processing of the GPC precursor is a highly regulated mechanism involving cellular enzymes and proteases [7]. The signal peptide of the GPC is myristoylated and forms an essential subunit of the mature G1-G2 complex [6]. Additionally, the GPC precursor undergoes cleavage to generate the GP1 and GP2 subunits, which are crucial for viral attachment and membrane fusion [8].
References:
[1] A. Capul, M. Perez, E. Burke, S. Kunz, M. Buchmeier, & J. Torre, "Arenavirus z-glycoprotein association requires z myristoylation but not functional ring or late domains", Journal of Virology, vol. 81, no. 17, p. 9451-9460, 2007. https://doi.org/10.1128/jvi.00499-07
[2] R. Iheozor-Ejiofor, L. Levanov, J. Hepojoki, T. Strandin, Å. Lundkvist, A. Plyusninet al., "Vaccinia virus-free rescue of fluorescent replication-defective vesicular stomatitis virus and pseudotyping with puumala virus glycoproteins for use in neutralization tests", Journal of General Virology, vol. 97, no. 5, p. 1052-1059, 2016. https://doi.org/10.1099/jgv.0.000437
[3] R. Pryce, W. Ng, A. Zeltina, Y. Watanabe, K. Omari, A. Wagneret al., "Structure-based classification defines the discrete conformational classes adopted by the arenaviral gp1", Journal of Virology, vol. 93, no. 1, 2019. https://doi.org/10.1128/jvi.01048-18
[4] E. Messina, J. York, & J. Nunberg, "Dissection of the role of the stable signal peptide of the arenavirus envelope glycoprotein in membrane fusion", Journal of Virology, vol. 86, no. 11, p. 6138-6145, 2012. https://doi.org/10.1128/jvi.07241-11
[5] K. Hastie, M. Zandonatti, L. Kleinfelter, M. Heinrich, M. Rowland, K. Chandranet al., "Structural basis for antibody-mediated neutralization of lassa virus", Science, vol. 356, no. 6341, p. 923-928, 2017. https://doi.org/10.1126/science.aam7260
[6] J. York, V. Romanowski, M. Lu, & J. Nunberg, "The signal peptide of the junín arenavirus envelope glycoprotein is myristoylated and forms an essential subunit of the mature g1-g2 complex", Journal of Virology, vol. 78, no. 19, p. 10783-10792, 2004. https://doi.org/10.1128/jvi.78.19.10783-10792.2004
[7] D. Burri, G. Pasqual, C. Rochat, N. Seidah, A. Pasquato, & S. Kunz, "Molecular characterization of the processing of arenavirus envelope glycoprotein precursors by subtilisin kexin isozyme-1/site-1 protease", Journal of Virology, vol. 86, no. 9, p. 4935-4946, 2012. https://doi.org/10.1128/jvi.00024-12
[8] D. Burri, J. Palma, S. Kunz, & A. Pasquato, "Envelope glycoprotein of arenaviruses", Viruses, vol. 4, no. 10, p. 2162-2181, 2012. https://doi.org/10.3390/v4102162