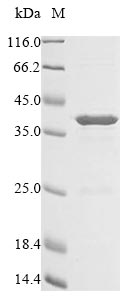
Recombinant Escherichia coli cea protein is an E.coli-expressed partial protein. Molecularly, this cea protein is characterized by N-terminal 10xHis tag and C-terminal Myc tag, internal Escherichia coli cea DNA fragment (1-304aa). Standard methods for recombinant cea protein expression comprise transfecting cells with DNA vectors that consist of specific templates and then cultures cells to translate and transcribe the cea protein production process. Typically, these cells are lysed to extract expressed proteins for more purification. The purity of this recombinant cea protein is 85%+ measured by SDS-PAGE.
cea, also known as colicin E1, is a bacteriocin produced by E. coli that acts against bacteria by forming a pore in the bacterial membrane, leading to membrane depolarization and cell death. The cobalamin translocator BtuB binds to the colicin E1, initiating the import of colicin E1. The drug-export protein TolC is essential for the import of colicin E1 across the outer membrane and periplasmic space. Treatment of cells harboring the plasmid ColE1 with chemicals that damage DNA or interfere with DNA synthesis can result in the production of colicin E1.