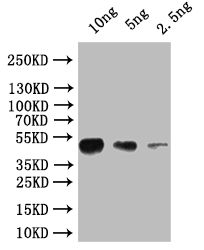
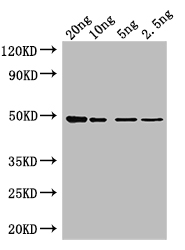
Drosophila Research-Related Products:
Exploring the Mysteries of Life Sciences
Drosophila, commonly known as the fruit fly, is a member of the Drosophila family within the order Diptera, with over 4,000 species identified globally. These tiny insects, measuring approximately 2 to 3 millimeters in length, are characterized by their yellowish to yellowish-brown bodies and, in many cases, large red compound eyes. The most renowned species, Drosophila melanogaster, is native to tropical and subtropical regions but has become a cosmopolitan species, mirroring the global spread of human populations.
Since the early 20th century, Drosophila has emerged as the preeminent model organism for genetic studies due to its distinctive biological traits. Its rapid reproduction rate, brief life cycle, and the simplicity of genetic manipulation make it an ideal candidate for swift experimentation. This allows researchers to observe genetic variations and delve deeper into the intricacies of genetic mechanisms.
Historically, the fruit fly has been instrumental in the field of genetics, contributing significantly to the accolades of six Nobel Prizes. Thomas Hunt Morgan pioneered the use of Drosophila as a model organism in the 1910s, uncovering the pivotal role of chromosomes in genetic inheritance. This groundbreaking work established the cornerstone of modern genetics and led to his Nobel Prize in Physiology or Medicine in 1933. Hermann Joseph Muller followed with a Nobel Prize in 1946 for his discovery that X-rays could induce genetic mutations. In 1995, the Nobel Prize was awarded to three scientists who utilized fruit flies to elucidate the genetic mechanisms controlling early embryonic development. Richard Axel and Linda Buck received the Nobel Prize in 2004 for their work on olfactory receptors and signal transduction pathways, with a significant portion of their research grounded in Drosophila studies. The 2011 Nobel Prize recognized Jules Hoffmann and Bruce Beutler for their discovery of the innate immune response activation mechanism, with Hoffmann's research incorporating Drosophila. In 2017, Jeffrey Hall, Michael Rosbash, and Michael Young were honored for their discovery of the molecular mechanisms governing circadian rhythms, with their research also leveraging the fruit fly as a model organism. These milestones underscore the indispensable role of fruit flies in genetics and broader biological research.
Fruit flies continue to be vital in the realms of developmental biology, neurobiology, behavioral genetics, and disease modeling, aiding scientists in uncovering a multitude of biological processes and disease mechanisms. CUSABIO provides a range of proteins and antibodies pertinent to fruit fly research, bolstering your endeavors in the scientific exploration of these remarkable insects.
● Fruit Fly Scientific Research-Related Proteins
Recombinant Drosophila melanogaster Stress-activated protein kinase JNK (bsk)
CSB-BP310094DLU
Recombinant Drosophila melanogaster Sterile alpha and TIR motif-containing protein 1
CSB-EP764228DLU1(M)
Recombinant Drosophila melanogaster GEO11329p1 (ITP)
CSB-MP3350DLU
Recombinant Drosophila melanogaster Nuclear RNA export factor 2 (nxf2), partial
CSB-BP016219DLU
| Product Name |
Code |
Target |
Source |
Tag Info |
| Recombinant Drosophila melanogaster 5-hydroxytryptamine receptor 2B (5-HT1B), partial |
CSB-EP010888DLU1 |
5-HT1B |
E.coli |
C-terminal 6xHis-tagged |
| Recombinant Drosophila melanogaster Barrier-to-autointegration factor (baf) |
CSB-YP892867DLU |
baf |
Yeast |
N-terminal hFc-tagged |
| Recombinant Drosophila melanogaster Spectrin beta chain (beta-Spec), partial |
CSB-EP251598DLU |
beta-Spec |
E.coli |
N-terminal 6xHis-tagged |
| Recombinant Drosophila melanogaster Protein bottleneck (bnk) |
CSB-EP328169DLU |
bnk |
E.coli |
N-terminal 6xHis-tagged |
| Recombinant Drosophila melanogaster Brain tumor protein (brat), partial |
CSB-EP839760DLU |
brat |
E.coli |
N-terminal 6xHis-tagged |
| Recombinant Drosophila melanogaster Stress-activated protein kinase JNK (bsk) |
CSB-BP310094DLU |
bsk |
Baculovirus |
C-terminal 9xHis-tagged |
| Recombinant Drosophila melanogaster Stress-activated protein kinase JNK (bsk) |
CSB-EP310094DLU |
bsk |
E.coli |
N-terminal 6xHis-tagged |
| Recombinant Drosophila melanogaster Bursicon (burs) |
CSB-EP894207DLU |
burs |
E.coli |
N-terminal GST-tagged |
| Recombinant Drosophila melanogaster Bursicon (burs) |
CSB-YP894207DLU |
burs |
Yeast |
N-terminal 6xHis-tagged |
| Recombinant Drosophila melanogaster Cyclin-dependent kinase 12 (Cdk12), partial |
CSB-EP894718DLU |
Cdk12 |
E.coli |
N-terminal 10xHis-tagged and C-terminal Myc-tagged |
| Recombinant Drosophila melanogaster Clathrin heavy chain (Chc), partial |
CSB-EP333472DLU |
Chc |
E.coli |
N-terminal 6xHis-tagged |
| Recombinant Drosophila melanogaster Sterile alpha and TIR motif-containing protein 1 (Ect4) (A386L,H392R,T433V,T434A,L595S,A596D,H599Q), partial |
CSB-EP764228DLU1(M) |
Ect4 |
E.coli |
N-terminal 6xHis-tagged |
| Recombinant Drosophila melanogaster Sterile alpha and TIR motif-containing protein 1 (Ect4), partial |
CSB-EP764228DLU1 |
Ect4 |
E.coli |
N-terminal 10xHis-tagged and C-terminal Myc-tagged |
| Recombinant Drosophila melanogaster Glyceraldehyde-3-phosphate dehydrogenase 1 (Gapdh1) |
CSB-EP362145DLU |
Gapdh1 |
E.coli |
N-terminal 6xHis-GST-tagged |
| Recombinant Drosophila melanogaster Probable insulin-like peptide 7 (Ilp7) |
CSB-BP895238DLU |
Ilp7 |
Baculovirus |
N-terminal 10xHis-tagged and C-terminal Myc-tagged |
| Recombinant Drosophila melanogaster GEO11329p1 (ITP) |
CSB-MP3350DLU |
ITP |
Mammalian cell |
C-terminal hFc-tagged |
| Recombinant Drosophila melanogaster GEO11329p1 (ITP) |
CSB-MP3350DLUh6 |
ITP |
Mammalian cell |
C-terminal hFc-Myc-tagged |
| Recombinant Drosophila melanogaster General odorant-binding protein lush (lush) |
CSB-EP517298DLU |
lush |
E.coli |
N-terminal 10xHis-tagged and C-terminal Myc-tagged |
| Recombinant Drosophila mauritiana Mariner Mos1 transposase (marinerT) |
CSB-EP745313DLT |
marinerT |
E.coli |
C-terminal 6xHis-tagged |
| Recombinant Drosophila melanogaster 28S ribosomal protein S10, mitochondrial (mRpS10) |
CSB-EP894684DLU |
mRpS10 |
E.coli |
N-terminal 6xHis-tagged |
● Fruit Fly Scientific Research-Related Antibodies
| Product Name |
Code |
Target |
Species Reactivity |
Tested Applications |
| beta-Spec Antibody |
CSB-PA251598XA01DLU |
beta-Spec |
Drosophila melanogaster (Fruit fly) |
ELISA, WB |
| brat Antibody |
CSB-PA839760XA01DLU |
brat |
Drosophila melanogaster (Fruit fly) |
ELISA, WB |
| CG8889-RA Antibody |
CSB-PA227598 |
CG8889-RA |
Drosophila melanogaster |
ELISA, WB |
| Chc Antibody |
CSB-PA333472XA01DLU |
Chc |
Drosophila melanogaster (Fruit fly) |
ELISA, WB |
| Cpr Antibody |
CSB-PA635658XA01DLU |
Cpr |
Drosophila melanogaster (Fruit fly) |
ELISA, WB |
| Dop1R1 Antibody |
CSB-PA334865XA01DLU |
Dop1R1 |
Drosophila melanogaster (Fruit fly) |
ELISA, WB |
| Ets21C Antibody |
CSB-PA326915XA01DLU |
Ets21C |
Drosophila melanogaster (Fruit fly) |
ELISA, WB |
| Fbxl4 Antibody |
CSB-PA064959 |
Fbxl4 |
Drosophila melanogaster |
ELISA, WB |
| FMRFaR Antibody |
CSB-PA893353XA01DLU |
FMRFaR |
Drosophila melanogaster (Fruit fly) |
ELISA, WB |
| FMRFaR Antibody |
CSB-PA893353XA11DLU |
FMRFaR |
Drosophila melanogaster (Fruit fly) |
ELISA, WB |
| ftz-f1 Antibody |
CSB-PA339334XA01DLU |
ftz-f1 |
Drosophila melanogaster (Fruit fly) |
ELISA, WB |
| Inx6 Antibody |
CSB-PA895180XA01DLU |
Inx6 |
Drosophila melanogaster (Fruit fly) |
ELISA |
| Nmdar1 Antibody |
CSB-PA634509XA01DLU |
Nmdar1 |
Drosophila melanogaster (Fruit fly) |
ELISA, WB |
| nos Antibody |
CSB-PA340723XA01DLU |
nos |
Drosophila melanogaster (Fruit fly) |
ELISA, WB |
| osk Antibody |
CSB-PA333049XA01DLU |
osk |
Drosophila melanogaster (Fruit fly) |
ELISA, WB |
| pho Antibody |
CSB-PA848344XA01DLU |
pho |
Drosophila melanogaster (Fruit fly) |
ELISA, WB |
| Piezo Antibody |
CSB-PA562252XA01DLU |
Piezo |
Drosophila melanogaster (Fruit fly) |
ELISA, WB |
| Prim1 Antibody |
CSB-PA630508XA01DLU |
Prim1 |
Drosophila melanogaster (Fruit fly) |
ELISA, WB |
| pum Antibody |
CSB-PA326575XA01DLU |
pum |
Drosophila melanogaster (Fruit fly) |
ELISA, WB |
| sfl Antibody |
CSB-PA894173XA01DLU |
sfl |
Drosophila melanogaster (Fruit fly) |
ELISA, WB |
| stau Antibody |
CSB-PA329523XA01DLU |
stau |
Drosophila melanogaster (Fruit fly) |
ELISA, WB |
| Tet Antibody |
CSB-PA145385XA01DLU |
Tet |
Drosophila melanogaster (Fruit fly) |
ELISA, WB |
| Tet Antibody |
CSB-PA145385XA11DLU |
Tet |
Drosophila melanogaster (Fruit fly) |
ELISA, WB |
Recent Advances in Drosophila Research:
As a crucial model organism, Drosophila has long been featured across various research fields. Finetti et al. (2020) demonstrated that monoterpenoids can act as bioinsecticides within Drosophila species and induce behavioral changes requiring functional Type I Trace Amine-Associated Receptor 1 (TAR1). In D. melanogaster, TAR1 is primarily expressed in specific brain regions, affecting triglyceride levels, food intake, and motor activity [1]. Skerlova et al.(2020) presented the crystal structure of the glutathione S-transferase Epsilon 14 in the fruit fly, providing insights into its function [2]. Landis et al. (2020) outlined the lifespan determination methods for D. melanogaster, emphasizing its importance as a model for aging research [3]. Delbare et al. (2020) highlighted the impact of microbiome interactions and mating on the transcriptome of D. melanogaster females [4]. Schwarz et al. (2020) revealed the invasion of the Tirant transposable element in D. melanogaster populations without inducing hybrid dysgenesis symptoms [5]. Additionally, Ekka et al. (2021) assessed the toxicity of silica-titania core-shell nanocomposites in D. melanogaster, underscoring the importance of understanding potential environmental impacts [6]. Wallace et al. (2021) reported the discovery of a DNA virus associated with D. melanogaster in Europe, offering insights into the antiviral immunity of arthropods [7]. Raji et al. (2021) provided empirical evidence of the total number of neurons in the brains of fruit flies and mosquitoes, highlighting the value of insect species as model systems for studying brain function [8]. Furthermore, Zhang et al. (2021) investigated a newly discovered criptivirus affecting the host pupal period and fertility of D. melanogaster, demonstrating complex interactions within ecosystems [9]. Biglou et al. (2021) provided an overview of the insulin signaling pathway in model organisms such as Drosophila, emphasizing the conserved function of the insulin signaling pathway across species [10]. These studies collectively underscore the importance of D. melanogaster as a versatile model organism in a diverse range of research fields.
References:
[1] Luca Finetti, Lasse Tiedemann, Xiaoying Zhang, et al. Monoterpenes Alter TAR1-driven Physiology in Drosophila Species", THE JOURNAL OF EXPERIMENTAL BIOLOGY, 2020.
[2] J. Skerlova, H. Lindstrom, B. Sjodin, et al. Crystal Structure of Drosophila Melanogaster Glutathione S-transferase Epsilon 14 in Complex with Glutathione and 2-methyl-2,4-pentanediol, 2020.
[3] Gary N Landis, Devon Doherty, John Tower, Analysis of Drosophila Melanogaster Lifespan", METHODS IN MOLECULAR BIOLOGY (CLIFTON, N.J.), 2020.
[4] Sofie Y. N. Delbare, Yasir H. Ahmed-Braimah, et al. Interactions Between The Microbiome and Mating Influence The Female's Transcriptional Profile in Drosophila Melanogaster, SCIENTIFIC REPORTS, 2020.
[5] Florian Schwarz, Filip Wierzbicki, Kirsten-André Senti, et al. Tirant Stealthily Invaded Natural Drosophila Melanogaster Populations During The Last Century, MOLECULAR BIOLOGY AND EVOLUTION, 2020.
[6] Basanti Ekka, Gyanaseni Dhar, Sumanta Sahu, et al. Removal of Cr(VI) By Silica-titania Core-shell Nanocomposites: In Vivo Toxicity Assessment of The Adsorbent By Drosophila Melanogaster, CERAMICS INTERNATIONAL, 2021.
[7] M. A. Wallace, K. A. Coffman, C. Gilbert, et al. The Discovery, Distribution and Diversity of DNA Viruses Associated with Drosophila Melanogaster in Europe, BIO.MICROBIOLOGY, 2021.
[8] Joshua I Raji, Christopher J Potter, The Number of Neurons in Drosophila and Mosquito Brains, PLOS ONE, 2021.
[9] Jiao Zhang, Fei Wang, Bo Yuan, et al. A Novel Cripavirus of An Ectoparasitoid Wasp Increases Pupal Duration and Fecundity of The Wasp's Drosophila Melanogaster Host", THE ISME JOURNAL, 2021.
[10] Sanaz G Biglou, William G Bendena, Ian Chin-Sang, An Overview of The Insulin Signaling Pathway in Model Organisms Drosophila Melanogaster and Caenorhabditis Elegans, PEPTIDES, 2021.



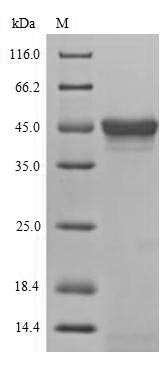
-SDS.jpg)