What is MAPK Signaling Pathway?
The mitogen-activated protein kinase (MAPK) cascade is a highly conserved module that is involved in various cellular functions, including cell proliferation, differentiation and migration. The pathway covers many proteins, including MAPK originally named ERK (extracellular signal-regulated kinases), which transmits signal by phosphorylates a neighboring protein, as like an "on" or "off" switch.
The Function of MAPK Signaling Pathway
The MAPK signaling pathway is essential in regulating many cellular processes including inflammation, cell stress response, cell differentiation, cell division, cell proliferation, metabolism, motility and apoptosis. The role of the MAPK pathway in cancer, immune disorders and neurodegenerative diseases has been well recognized.
The Processes of MAPK Signaling Pathway
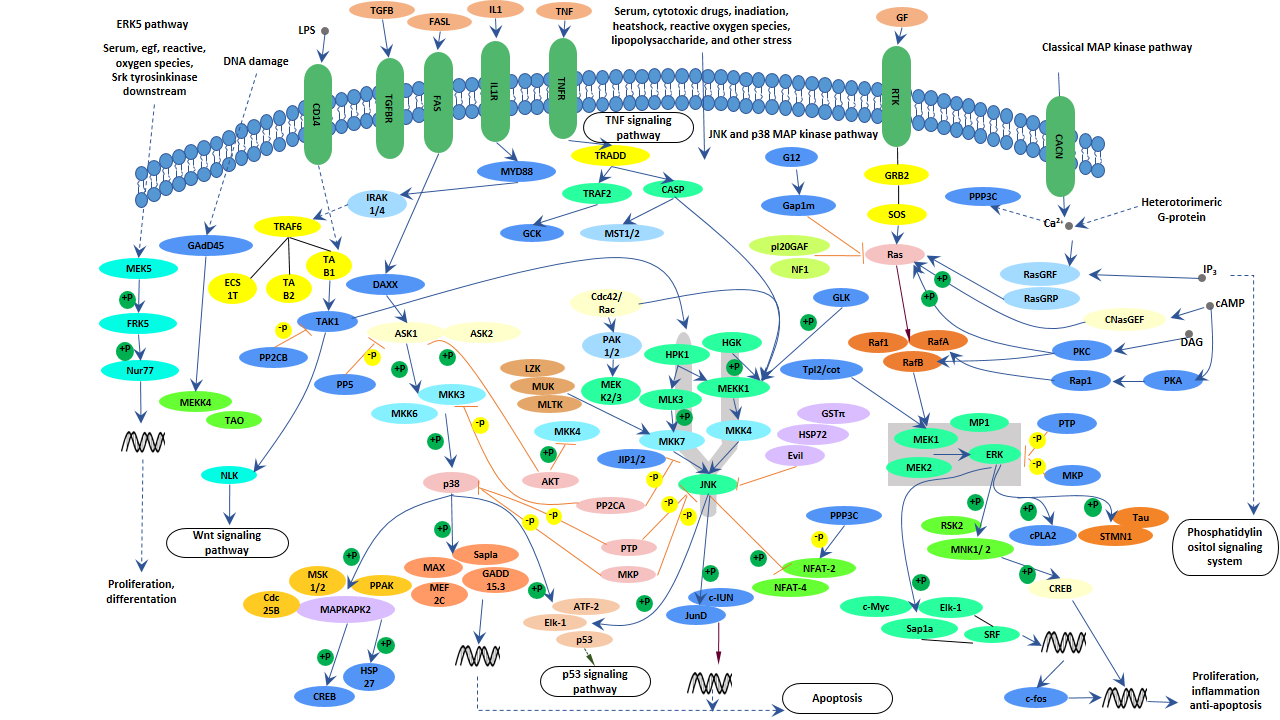
Mammals express at least four distinctly regulated groups of MAPKs, extracellular signal-related kinases (ERK)-1/2, Jun amino-terminal kinases (JNK1/2/3), p38 proteins (p38alpha/beta/gamma/delta) and ERK5 that are activated by specific MAPKKs: MEK1/2 for ERK1/2, MKK3/6 for the p38, MKK4/7 (JNKK1/2) for the JNKs, and MEK5 for ERK5.
But each MAPKK can be activated by more than one MAPKKK, increasing the complexity and diversity of MAPK signaling. Presumably each MAPKKK confers responsiveness to distinct stimuli. As shown in the following picture, Classical mitogen-activated protein kinase (MAPK) pathway activation begins at the cell membrane where small GTPases and various protein kinases phosphorylate MAPKKKs. Subsequently, MAPKKKs directly phosphorylate and activate MAPKKs. Once phosphorylated, activated MAPKs phosphorylate numerous cytoplasmic substrates and ultimately modulate transcription factors that drive context-specific gene expression.
Although MAPK signaling cascades are depicted as simple linear, unidirectional groups of protein kinases, the pathway is highly complex, actually. A large degree of cross-talk within the MAPK cascades and other signaling networks exists. For example, interactions between mediators of the MAPK, PI3K networks, NFκB pathway and JAK-STAT pathway are well documented.
The MAPK Signaling Pathway and Cancer
The MAPK signaling pathway is closely related to cell proliferation and differentiation. Continuous activation of MAPK signals is found in many malignant tumors. Cell proliferation contributes largely to tumorigenesis. And tumorous metastasis and invasion are lethal causes. Among the mammalian MAPK pathways, the ERK pathway is the best-studied and is deregulated in about one-third of human cancers. The ERK1/2 pathway plays an important role in proliferation, differentiation, metastasis, and invasion of tumor cells. Wen-Horng Wang, et al. found that the hepatitis b virus x protein inactivated p53 gene through the p38/MAPK pathway, thus inducing the primary liver cancer (PLC). Kim MS, et al. demonstrated that p38 MAPK is a key signal molecule in breast cancer cells' invasion and metastasis induced by Ras. And many studies showed that the p38 MAPK pathway exerts important regulatory functions in MMPs (matrix metalloproteinases) produced by extracellular stimuli. MMPs are a type of proteolytic enzyme tightly associated with tumorous invasion and migration. Besides, the p38 MAPK pathway promotes tumor angiogenesis necessary to maintain tumor cell growth.
Cancer is often exposed to a variety of stress conditions such as oxygen deprivation and inflammation. Many MAPK pathways are involved in stress signaling. Therefore, some kinases are activated by stress in cancer in response to inflammation, DNA damage, and apoptosis. Hypoxic states are widespread in solid tumors. The hypoxic signal stimulates the activation of MKP-1. MKP-1 further activates SAPK/JNKs, which activates c-Jun. Active c-Jun promotes multiple downstream genes transcription that is important for cancer cells' proliferation and survival.
Aberrations in MAPK signaling influences most processes of carcinogenesis, and are crucial for the development and progression of cancer. Most cancer-associated lesions such as overexpression of receptor tyrosine kinases, activating mutations in receptor tyrosine kinases, sustained autocrine or paracrine production of activating ligands, Ras mutations and B-Raf mutations result in constitutive activation of ERK signaling. JNK activity and phosphorylation of c-Jun has been reported to play a critical role in Ras-induced tumorigenesis.





