Flu Season Returns: Understanding the Influenza Virus
As flu season arrives on schedule, our battle against this familiar foe has entered a new phase. Influenza viruses, particularly their constantly mutating surface proteins hemagglutinin (HA) and neuraminidase (NA), remain key targets of global public health surveillance. Current epidemiological monitoring shows ongoing influenza activity, while the scientific community continues to advance its understanding and intervention strategies.
On one hand, fundamental research is deepening our comprehension of the microscopic world of influenza viruses. The pathogenic core of influenza lies in its segmented RNA genome and the intricate interactions between its encoded key proteins (such as HA, NA, M2, etc.) and host cells. Recent studies reveal that viral invasion is far from a simple "lock-and-key" mechanism but more akin to a precise "cellular hijacking," with mechanisms more sophisticated than previously understood. This underscores the importance of basic research targeting these conserved sites (such as nucleoprotein NP and the polymerase complex PB1/PB2/PA) during the viral life cycle.
On the other hand, response strategies are also breaking new ground. A novel broad-spectrum antiviral strategy recently proposed by Chinese research teams, designed similarly to delivering a "Trojan horse" into the virus, aims to simultaneously disrupt multiple key targets. This provides a new approach for developing next-generation potent, long-lasting, and drug-resistant therapies. These cutting-edge advances rely on a thorough understanding of the structure and function of various influenza viral protein targets.
Therefore, during the peak flu season, in addition to relying on vaccination and daily protective measures, sustained and in-depth fundamental research is the cornerstone of building future defenses. This article aims to systematically review the classification, structure, pathogenic mechanisms, and current prevention and treatment strategies of influenza viruses, hoping to provide clear background knowledge for researchers in related fields. In-depth study of viral protein targets is not only the key to understanding their transmission and mutation but also the starting point for developing new diagnostic tools, antiviral drugs, and vaccines. The battle between humans and influenza is a protracted one, and each scientific advancement gives us an additional edge in this ongoing struggle.
Classification
Influenza viruses are mainly divided into three species: influenza A virus, influenza B virus, and influenza C virus. Only type A and B are clinically relevant to humans. Although both viruses can cause human infection, genera A typically leads to widespread illness and epidemics.
-
Influenza A virus
It was successfully isolated in 1933. Influenza A virus is commonly carried by animals such as birds, pigs, and is a pathogenic agent of zoonotic infections. Only influenza type A viruses are known to have caused pandemics. Influenza A virus undergoes an antigenic shift, which is a sudden change in antigenicity resulting from genetic recombination between viral strains, producing a new virus subtype. Based on the properties of the surface proteins hemagglutinin (H) and neuraminidase (N), influenza A viruses are subclassified and named by combining the H and N numbers, such as A(H1N1), A(H3N2).
-
Influenza B virus
Type B was obtained in 1940. It is only found in humans and usually causes milder symptoms than type A virus. Although it can trigger seasonal illness and human epidemics, influenza B virus has not caused a pandemic. Influenza B viruses are further classified into two lineages: B/Yamagata and B/Victoria.
-
Influenza C virus
Type C infections generally cause mild illness and are not thought to cause human flu epidemics.
Structure
Influenza A and B viruses are virtually indistinguishable by electron microscopy. They are typically spherical with a diameter of about 100 nm, but sometimes are filamentous with a length usually exceeding 300 nm. The outermost layer of influenza virus particles is the host cell-deriving envelope. The envelope is embedded with glycoprotein spikes of hemagglutinin (HA) and neuraminidase (NA), protruding from the envelope. Both HA and NA determine the subtypes of the influenza virus. The ratio of HA to NA is about 4:1. Less matrix ion channels M2 spans the lipid envelope. The matrix protein M1 is beneath the envelope and encapsulates the core of the virion.
Inward the virion is the viral genome composed of 8 pieces of single-stranded RNA (only type A and B). The RNA, coated with nucleoprotein and heterotrimeric RNA-dependent RNA polymerase (three subunits: PB1, PB2, and PA), is assembled into a helical ribonucleoprotein (RNP) complex. Nuclear export protein (NEP) is also found in the interior of the virion.
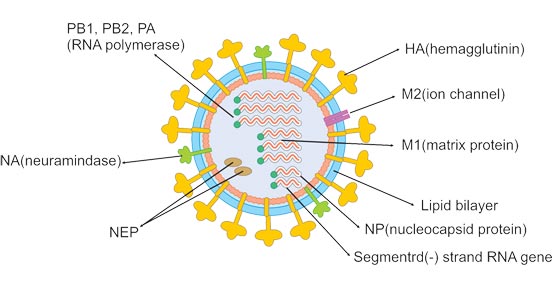
Figure 1.
| Target |
Protein |
Description |
| HA |
Hemagglutinin |
The HA protein is processed into HA1 and HA2 by serine proteases during viral replication, which gives virus infectivity. The HA2 is involved in the fusion of viral envelope with host cell membrane, while the HA1 contains the receptor binding and antigenic sites. It is also an antigen that can be recognized by the immune system. |
| NA |
Neuraminidase |
It is capable of destroying receptor, cleaving terminal sialic acid residues from cell-surface glycoproteins and gangliosides to release offspring virus from the host cell. Additionally, the NA also removes sialic acid residues from the virus envelope, blocking the aggregation of viral particles to enhance infectivity. |
| M1 |
matrix protein M1 |
M1 interacts with both viral RNA and nucleoprotein, bringing them together within the RNP complex. It also associates with NEP, mediating the M1-RNP export via nucleoporins into the cytoplasm. |
| M2 |
matrix ion channels M2 |
It is the target of the amantadine class of anti-influenza drugs, which inhibit ion channel activity and prevent virus from unshelling. As a surface protein, it can be a vaccine component. |
| NEP/NS |
Nuclear export protein |
NEP, together with M1, is involved in the translocation of M1-RNP into the cytoplasm. |
| NP |
Nucleoprotein |
Viral RNA is packaged with nucleoprotein into a helical ribonucleoprotein complex. |
| RNP |
Ribonucleoprotein |
The RNA, coated with nucleoprotein and heterotrimeric RNA-dependent RNA polymerase (three subunits: PB1, PB2, and PA), assembls into a helical ribonucleoprotein (RNP) complex. |
See All Related Proteins of Influenza-Virus
The Influenza Pandemic Event
In 1918-1920 Spanish Flu, more than 50 million people died of H1N1 influenza worldwide. About 1.5 to 2 million people died in the 1957-1958 Asian flu pandemic, which was caused by the H2N2 influenza A subtype. The H3N2 led to the 1968-1969 Hong Kong Flu, causing 1million deaths. The bird flu panic of 2006 was caused by a strain of Influenza A. The swine flu of 2009 caused a great deal of widespread sickness and fear. This was a new strain of influenza A called H1N1 and was carried by pigs but quickly spread through humans. The 2009 Swine Flu was spread to 208 countries and caused approximately 18000 deaths. The pandemic of H7N3 avian influenza in Mexico in late 2012 was the largest ever outbreak in poultry in North America, with 20 million birds killed. And influenza has become a typical seasonal flu and causes about 290000 to 650000 deaths annually around the world.
Transmission
Influenza is a typical seasonal diseases and frequently occurs in spring and winter. There are three transmission routes of influenza virus.
-
Through aerosol infection
The influenza virus is mainly transmitted by aerosol infection. For example, large droplets (larger 5 μm in diameter) produced when talking, coughing, or sneezing enters the mucous membrane through short-distance contact.
-
Through aerogenic transmission
Propagation may occur in tiny droplet cores (smaller 5 μm in diameter) that can stay in the air for longer periods.
-
Through direct contact
Direct contact with virus-contaminated surfaces (such as handshaking) and subsequent oral and nasal contact can cause transmission. Isolation and identification of virus
Related Proteins
| Target |
Product Name |
Source |
Tag Info |
Product Code |
| BM2 |
Recombinant Influenza B virus Matrix protein 2 (BM2) (I105V,R109H) |
in vitro E.coli expression system |
C-terminal 10xHis-tagged |
CSB-CF5657GWB(M) |
| HA |
Recombinant Influenza A virus Hemagglutinin (HA) |
E.coli |
N-terminal 6xHis-SUMO-tagged |
CSB-EP356317IDG |
| HA |
Recombinant Influenza A virus Hemagglutinin (HA) |
Yeast |
N-terminal 6xHis-tagged |
CSB-YP356317IDG |
| HA |
Recombinant Influenza A virus Hemagglutinin (HA) (X347S,X348S,X509S,X538S), partial |
Yeast |
N-terminal 6xHis-tagged |
CSB-YP356048IBA |
| HA |
Recombinant Influenza A virus Hemagglutinin (HA), partial |
E.coli |
N-terminal 10xHis-tagged |
CSB-EP3563GMC |
| HA |
Recombinant Influenza A virus Hemagglutinin (HA), partial |
Mammalian cell |
C-terminal hFc-tagged |
CSB-MP3563GMC |
| HA |
Recombinant Influenza A virus Hemagglutinin (HA), partial |
Mammalian cell |
C-terminal 10xHis-tagged |
CSB-MP3563GMC1 |
| HA |
Recombinant Influenza A virus Hemagglutinin (HA), partial |
Mammalian cell |
C-terminal 10xHis-tagged |
CSB-MP3563GMCd7 |
| HA |
Recombinant Influenza A virus Hemagglutinin (HA), partial |
E.coli |
N-terminal 6xHis-SUMO-tagged |
CSB-RP189374BA |
| HA |
Recombinant Influenza A virus Hemagglutinin (HA), partial |
E.coli |
N-terminal 6xHis-SUMO-tagged |
CSB-EP607773IER |
| HA |
Recombinant Influenza A virus Hemagglutinin (HA), partial |
E.coli |
N-terminal 6xHis-SUMO-tagged |
CSB-EP714917IEX |
| HA |
Recombinant Influenza A virus Hemagglutinin (HA), partial |
E.coli |
N-terminal 6xHis-SUMO-tagged |
CSB-EP721113IFG |
| HA |
Recombinant Influenza B virus Hemagglutinin (HA), partial |
E.coli |
C-terminal 6xHis-tagged |
CSB-EP5610GWB |
| HE |
Recombinant Influenza C virus Hemagglutinin-esterase-fusion glycoprotein (HE) , partial |
E.coli |
N-terminal 10xHis-tagged |
CSB-EP362283IKF |
| HE |
Recombinant Influenza C virus Hemagglutinin-esterase-fusion glycoprotein (HE), partial |
Yeast |
N-terminal 6xHis-tagged |
CSB-YP365951IKD |
| HE |
Recombinant Influenza C virus Hemagglutinin-esterase-fusion glycoprotein (HE), partial |
Baculovirus |
N-terminal 10xHis-tagged |
CSB-BP362283IKF |
| M |
Recombinant Influenza A virus Matrix protein 1 (M) |
E.coli |
N-terminal 10xHis-tagged and C-terminal Myc-tagged |
CSB-EP389592ILS |
| M |
Recombinant Influenza A virus Matrix protein 2 (M) |
in vitro E.coli expression system |
N-terminal 10xHis-tagged |
CSB-CF356944IFZ |
| M |
Recombinant Influenza A virus Matrix protein 2 (M) |
in vitro E.coli expression system |
N-terminal 6xHis-tagged |
CSB-CF389902ILU |
| M |
Recombinant Influenza A virus Matrix protein 2 (M) |
in vitro E.coli expression system |
N-terminal 10xHis-tagged |
CSB-CF389902ILUb0 |


